![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
19 Cards in this Set
- Front
- Back
|
addition reactions
|
occur when two reactants add together to form a single product with no atoms "left over"
|
|
|
elimination reactions
|
occur when a single reactant splits into two products, often with formation of a small molecule such as water or HBr.
may involve a catalyst |
|
|
substitution reaction
|
occurs when two reactants exchange parts to give two new products
|
|
|
rearrangement reaction
|
occurs when a single reactant undergoes a reorganization of bonds and atoms to yield an isomeric product
|
|
|
reaction mechanism
|
an overall description of how a reaction occurs
describes in detail exactly what takes place at each stage of a chemical transformation--which bonds are broken and in what order, which bonds are formed and in what order, and what the relative rates of the steps are |
|
|
All chemical reactions involves:
|
bond breaking and bond making
|
|

bond breaking
show movement of one electron in the symmetrical process |

|
|
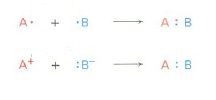
show e- movement in the formation of a bond
|
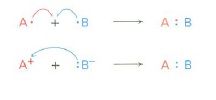
|
|
|
radical reactions
|
processes that involve symmetrical bond-breaking and bond-making
|
|
|
radical
|
often called a "free radical"
A neutral chemical species that contains an odd number of electrons and thus has a single, unpaired electron in one of its orbitals |
|
|
polar reactions
|
processes that involve unsymmetrical bond-breaking and bond-making
|
|
|
a radical is highly reactive because:
|
it contains an atom with an odd number of electrons (usually seven) in its valence shell
|
|
|
prostaglandins
|
a large class of molecules found in virtually all body tissues and fluids
|
|
|
polar reactions occur because of:
|
the electrical attraction between positive and negative centers on functional groups in molecules
|
|
|
metals bonded to carbon are _____ EN
|
less electronegative
So, a carbon atom bonded to a metal has a partial negative charge |
|

Draw these
|
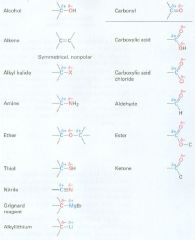
|
|
|
are curved, full-headed arrow, shows:
|
where electrons move when reactant bonds are broken and product bonds are formed
|
|
|
nucleophile
|
a substance that is "nucleus-loving"
A nucleophile has a negatively polarized, electron-rich atom and can form a bond by donating a pair of electrons to a positively polarized, electron-poor atom The nucleophile may be either neutral or negatively charged. e.g., ammonia, water, hydroxide ion, chloride ion |
|
|
electrophile
|
"electron loving"
An electrophile has a positively polarized, electron-poor atom and can form a bond by accepting a pair of electrons from a nucleophile Can be either neutral or positively charged. E.g., acids (H+ donors), alkyl halides, carbonyl compounds |

