![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
|
The subunit that serves as the building block of a polymer.
Are connected by a reaction in which two molecules are covalently bonded to each other through loss of a water molecule; one molecule proves a hydroxyl group (-OH), other provides a hydrogen (-H), which makes up a polymer. |
Monomers
|
|
|
A long molecule consisting of many similar of identical monomers linked together.
Are disassembled to monomers by hydrolysis, process of breaking bonds by the addition of a water molecule, (-H) from water attaches to one monomer and a (-OH) attaches to the adjacent monomer. One water molecule is required to hydrolyze each connected pair of monomers - when breaking down a polymer. |
Polymers
|
|
|
A giant molecule formed by the joining of smaller molecules, usually by a condensation reaction.
Polysaccharides, proteins, and nucleic acids are macromolecules. |
Macromolecules
|
|
|
A chemical reaction in which two molecules covalently bond to each other with the removal of a water molecule.
|
Dehydration reaction
|
|
|
A reaction in which two molecules become covalently bonded to each other through the loss of a small molecule, usually, in which case it is also called dehydration reaction.
|
Condensation reaction
|
|
|
A chemical process that lyses, or splits, molecules by the addition of water in disassembly of polymers to monomers.
|
Hydrolysis
|
|
|
A macromolecule serving as a catalyst, a chemical agent that changes the rate of a reaction without being consumed by the reaction.
|
Enzyme
|
|
|
Most common monosaccharide and is central importance in the chemistry of life.
Structure has a (>C=O) a carbonyl group and multiple hydroxyl groups (-OH). In aqueous solutions, it and most other sugars form rings. In a process known as cellular respiration, cells extract energy in a series of reactions starting with glucose molecules (which is a major nutrient for cells). |
Glucose
|
|
|
Depending on the location of the carbonyl group, a sugar is either.... ?
|
aldose (aldehyde sugar) or a ketose (ketone sugar).
Example: glucose, is an aldose; fructose (a structural isomer of glucose, is a ketose. |
|
|
The size of the carbon skeleton, which ranges from three to seven carbons long and fall into one of what three groups?
|
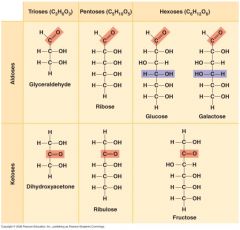
Trioses (tree-carbon sugars)
Pentoses (five-carbon sugars) Hexoses (six-carbon sugars) |
|
|
Whether a sugar is is aldoses (aldehyde sugar) or ketoses (ketone sugar) depends on the location of what?
|
Carbonyl (>C=O)
|
|
|
Another variation that determines a sugars classification is what?
|
The spatial arrangement around asymmetric carbons (for example, the portions of glucose (H-C-OH) and galactose (HO-C-H)
|
|
|
A sugar (monosaccharide) or one of its dimers (disaccharide) or polymers (polysaccharides).
Monosaccharide: The simplest carbohydrate, active alone or serving as a monomer for disaccharides and polysaccharides. Also known as simple sugars, monosaccharides have molecular formulas that are generally some multiple of CH2O. |
Carbohydrate
|
|
|
A double sugar, consisting of two monosaccharaides joined by a glycosidic linkage formed during dehydration synthesis.
|
Disaccharide
|
|
|
A covalent bond formed between two monosaccharides by a dehydration reaction.
|
Glycosidic linkage
|
|
|
A polymer of many monosaccharides, formed by dehydration reactions.
|
Polysaccharide
|
|
|
A storage polysaccharide in plants, consisting entirely of glucose monomers joined by glycosidic linkages.
|
Starch
|
|
|
An extensively branched glucose storage polysaccharide found in the liver and muscle of animals; the animal equivalent of starch.
|
Glycogen
|
|
|
A structural polysaccharide of plant cell walls, consisting of glucose monomers joined by Beta glycosidic linkages.
|
Cellulose
|
|
|
A structural polysaccharide, consisting of amino sugar monomers, found in many fungal cell walls and in the exoskeletons of all arthropods.
|
Chitin
|
|
|
Examples of disaccharide:
a) Glycosidic linkage, a covalent bond between two glucose molecules forms disaccharide called? b) The monomers, glucose and fructose bond to form the most prevalent disaccharide called? |
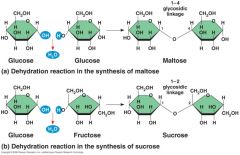
Glucose and Glucose - Maltose
Glucose and Fructose - Sucrose |
|
|
The carbonyl group in the following make them what type of sugar?
Glyceraldehyde Ribose Glucose Galactose |
Aldose (aldehyde sugars)
|
|
|
The carbonyl group in the following make them what type of sugar?
Dihydroxyacetone. Ribulose Fructose |
Ketose (ketone sugar)
|
|
|
Polysaccharides architecture and function are determined by its sugar monomers and by the positions of its glycosidic linkages and means what?
|
It means that they will be in one of two types of polysaccharides:
Some will serve as storage material, hydrolyzed as needed to provide sugar for cells (storage polysaccharides). Others will serve as building material for structures that protect the cell or the whole organism (structural polysaccharides). |
|
|
Both plants and animals store sugars for later use in the form of what?
|
Storage polysaccharides
|
|
|
What is starch?
|
Storage polysaccharide stored by plants.
It's a polymer of glucose monomers, as granules within cellular structures know as plastids, which include chloroplast. |
|
|
What is the simplest form of starch and which is unbranched (1-4 linkages)?
|
Amylose
|
|
|
What is the more complex starch and which is a branched polymer (1-6 linkages at the branch points)?
|
Amylopectin
|
|
|
Most animals, including humans, have ______ that can _____ plant starch, making glucose available as a nutrient for cells.
|
Enzymes, used to hydrolyze the plant starch
|
|
|
What is glycogen?
|
Storage ploysaccharide that is stored by animals.
It's a polymer of glucose that is like amylopectin but more extensively branched. |
|
|
It's stored mainly in the _____ and _____ ; hydrolysis of glycogen in these cells release glucose when the demand for sugar increases. Is this stored fuel sustained for long or short periods of time in humans?
|
Liver and muscles, sustained for short periods of time and depletes in about da day unless replenished with food.
|
|
|
What are some of the characteristics of Lipids?
|
Are generally NOT thought of as macromolecules.
One of a group of compounds, including fats, phospholipids, and steroids, that mix poorly, if at all, with water. Vary in form and function – waxes and certain pigments. Most biological important types are fats, phospholipids, and steroids. |
|
|
Fat is a lipid consisting of three fatty acids linked to one glycerol molecule, also called what?
|
Triacylglycerol or triglyceride.
|
|
|
Lipid that is a long carbon chain carboxylic acid, vary in length and in the number and location of double bonds; three of these linked to a glycerol molecule form a fat molecule, also known as a triacylglycerol or triglyceride.
|
Fatty acid:
|
|
|
Triacylglycerol has three fatty acids linked to one glycerol molecule; also called what?
|
A fat or triglyceride.
|
|
|
What is the name of this lipid that is a fatty acid in which all carbons in the hydrocarbon tail are connected by single bonds, thus maximizing the number of hydrogen atoms that are attached to the carbon skeleton?
|
Saturated fatty acid
|
|
|
A fatty acid possessing one or more double bonds between the carbons in the hydrocarbon tail. Such bonding reduces the number of hydrogen atoms attached to the carbon skeleton.
|
Unsaturated fatty acid
|
|
|
An unsaturated fat containing one or more trans double bonds.
|
Trans fats
|
|
|
A lipid made up of glycerol joined to two fatty acids and a phosphate group. The hydrocarbon chains of the fatty acids act as nonpolar, hydrophobic tails, while the rest of the molecule acts as a polar, hydrophilic head.
|
Phospholipid
|
|
|
Phospholipids form what that fucntion as what?
|
They form bilayers that function as biological membranes.
|
|
|
A type of lipid characterized by a carbon skeleton consisting of four rings with various chemical groups attached.
|
Steroid
|
|
|
A steroid that forms an essential component of animal cell membranes and acts as a precursor molecule for the synthesis of other biologically important steroids, such as hormones.
|
Cholesterol
|
|
|
A chemical agent that increases the rate of reaction without being consumed by the reaction.
|
Catalyst
|
|
|
A polymer (chain) of many amino acids linked together by peptide bonds.
Protein: A functional biological molecule consisting of one or more polypeptides folded and called into a specific three-dimensional structure. Amino acid: An organic molecule possessing both carboxyl and amino groups. Amino acids serve as the monomers of polypeptides. Peptide bond: The covalent bond between the carboxyl group on one amino acid and the amino group on another, formed by a dehydration reaction. |
Polypeptide
|
|
|
How many levels of protein structure are there?
|
4
|
|
|
The level of protein structure referring to the specific sequence of amino acids.
|
Primary structure
|
|
|
Secondary structure, which the localized, repetitive coiling or folding of the polypeptide backbone of a protein due to hydrogen bond formation between constituents of the backbone. What are the two forms of secondary structure?
|
Alpha helix: A spiral shape constituting one form of the secondary structure of proteins arising from a specific pattern of hydrogen bonding.
Beta pleated sheet: One form of secondary structure of proteins in which the polypeptide chain folds back and forth. Two regions of the chain lie parallel to each other and are held together by hydrogen bonds. |
|
|
Tertiary structure: Irregular contortions of a protein molecule due to interactions of side chains involved in what bypes of interactions, bonds, and bridges?
|
They are hydrophobic interactions, ionic bonds, hydrogen bonds, and disulfide bridges.
|
|
|
A type of weak chemical bond formed when molecules that do not mix with water coalesce to exclude water.
|
Hydrophobic interaction:
|
|
|
A strong covalent bond formed when the sulfur of one cysteine monomer bonds to the sulfur of another cysteine monomer.
|
Disulfide bridge:
|
|
|
The particular shape of a complex, aggregate protein, defined by the characteristic three-dimensional arrangement of tis constituent subunits, each a polypeptide is which level of protein structure?.
|
Quaternary structure
|
|
|
In proteins, a process in which a protein unravels and loses its native shape, thereby becoming biologically inactive; in DNA, the separation of the two strands of the double helix. Denaturation occurs under extreme (noncellular) conditions of pH, salt concentration, and temperature.
|
Denaturation:
|
|
|
A protein molecule that assists in the proper folding of other proteins.
|
Chaperonin:
|
|
|
A technique that depends on the diffraction of an X-ray beam by the individual atoms of a crystallized molecule to study the three-dimensional structure of the molecule.
|
X-ray crystallography:
|
|
|
A discrete unit of hereditary information consisting of a specific nucleotide sequence in DNA (or RNA, in some viruses).
|
Gene:
|
|
|
Nucleic acid: A polymer (polynucleotide) consisting of many nucleotide monomers; serves as a blueprint for proteins and, through the actions of proteins, for all cellular activities. What are the two types?
|
The two types are DNA and RNA.
|
|
|
Your body contains thousands of different types of proteins, each with a specific function. A protein's function is largely determined by its _____.
|
shape
electrical charge elemental composition size |
|
|
The primary structure of a protein is like the arrangement of beads on a string. In this analogy, the beads are _____, of which there are _____ different kinds.
|
amino acids ... 20
amino acids ... 26 nucleotide bases ... 4 nucleotide bases ... 20 |
|
|
A protein's alpha helices and beta sheets fold together to create an overall shape at the _____ level of protein structure.
|
first
second third fourth |
|
|
What happens to the shape and function of a protein if one of the amino acids is replaced with a different type of amino acid?
|
The protein will unravel and become entirely nonfunctional.
It depends on the role of the amino acid that is altered. One amino acid might be replaced with no measurable effect on the protein's function; replacing another might cause a total loss of function. The protein will remain unchanged; several amino acids would have to be altered to have any effect on protein function. The protein's fourth-level structure will be damaged. |
|
|
Large proteins, like DNA polymerase and hemoglobin, are often composed of several polypeptides that are linked together. The _____ level of protein structure describes how the polypeptides are joined to create a larger complex.
|
first
second third fourth |

