![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
3 Cards in this Set
- Front
- Back
|
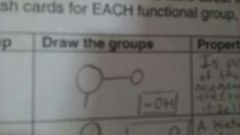
HYDROXYL Properties & Naming of molecules |
Properties: -Is polar as a result of the electronegative oxygen atom drawing electrons toward itself. -Atttacts water molecules, helping dissolve organic compounds such as sugars.
Naming of molecules: -Alcohol; name ends on -ol
|
|
|
CARBOXLY (type 1) Properties & Naming molecules |
Properties: -A ketone and an aldehyde may be structural isomers with different properties, as is the case for acetone and propanal.
Naming & molecules: -Ketones if the carboxly group is within a carbon skeleton. -Albehydes if the carboxly group is at the end of the carbon skeleton. |
|
|
Draw the groups |
|

