![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
|
Polar molecule |
The opposite ends have opposite charges |
|
|
Four emergent properties of water |
Cohesive behavior, ability to moderate tempurate, expansion upon freezing, versatility as a solvent |
|
|
Cohesion |
When Hydrogen bonds hold water molecules together |
|
|
Adhesion |
An attraction between different substances. Ex. Water and plant cells |
|
|
Surface tension |
The measure of how hard it is to stretch or break the surface of a liquid. It is related to cohesion |
|
|
Moderation of temperature by water |
Water absorbs hear from warmer air and releases stored heat to cool air. Water can absorb or release a large amount of heat with only a slight change in its own temperature. |
|
|
Kinetic energy |
The energy of motion |
|
|
Thermal energy |
The total kinetic energy associated with random movement of atom or molecules (matters volume) |
|
|
Temperature |
The average kinetic energy of the molecules in a body of matter |
|
|
Heat |
The transfer of thermal energy from one body of matter to another |
|
|
Calorie |
The amount of heat required to raise the temperature of 1g of water |
|
|
Joul |
Is another unit of energy where 1J= .239 calories. Or 1cal=4.184J |
|
|
Specific heat |
The amount of heat that must be absorbed or loft for 1g of that substance to change its temperature. |
|
|
Evaporation |
Transformation of a substance from liquid to gas |
|
|
Heat of vaporization |
The heat a liquid must absorb for 1g to be converted to gas |
|
|
Evaporative cooling |
The process of a liquid evaporates it's remaining surface cools |
|
|
Evaporative cooling |
Helps stabilize temperatures in organisms and bodies of water |
|
|
Solution |
A liquid that is homogenous mixture of substances |
|
|
Solvent |
The dissolving agent of a solution |
|
|
Solute |
Substance that is dissolved |
|
|
Aqueous solution |
One in which water is the solvent |
|
|
Hydration shell |
When an ionic compound is dissolved in water each ion is surrounded by a sphere of water molecules called a hydration compound. |
|
|
Hydrophillic |
Substance that has an affinity for water |
|
|
Hydrophobic |
Substance is one that does not have an affinity for water |
|
|
Molecular mass |
The sum of all masses of all atoms in a molecule |
|
|
1mol |
6.02 x 10^23 |
|
|
Avocados number |
6.02 x 10^23 daltons=1g |
|
|
Molarity |
The number of moles of solute per liter of solution |
|
|
Hydrogen (H+) |
The hydrogen atom leaves it's electron behind and is transferred as a proton |
|
|
Hydronium |
The molecule with the extra proton is now a hydronium ion (H+) |
|
|
Hydroxide ion OH- |
The molecule that lost the proton |
|
|
Water in a state of dynamic equilibrium (pic) |

|
|
|
Though statistically rare, the dissociation of water molecules has a great effect on organisms |
In pure water 554 million water molecules dissociated |
|
|
Concentrations of H+ OH- |
Are equal in water |
|
|
Acid |
Any substance that increases the H+ concentration of a solution |
|
|
Base |
Any substance that reduces the H+ concentration of a solution |
|
|
Examples of pH levels (pic) |
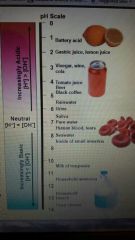
|
|
|
Buffers |
Substances that minimize changes in concentrations of H+ and OH- in a solution |
|
|
Fossil fuels |
CO2 is a main product of fossil fuel combustion. 25% of human generated CO2 is absorbed by the oceans. |
|
|
Ocean acidification |
Yhe proccess in which CO2 is dissolved in sea water forms carbon acid |

