![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
Covalent compounds are formed when a _____ bonds with one or more _____.
|
nonmetal
nonmetals |
|
|
_____, although a metal, will form covalent bonds with nonmetals.
|
Hydrogen
|
|
|
The first name of a covalent compound is the name of the _____ _____ in the compound.
|
first element
|
|
|
With covalent compounds, when are numerical prefixes used for the first name of the element?
|
only if there are two or more atoms of that element in the compound
|
|
|
The last name of a covalent compound is the name of the _____ _____ and ends in the letters _____.
|
second element
ide |
|
|
Name the prefixes for the first names of covalent compounds that are used for the numbers 1 -10.
|
1 = NOT USED
2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca |
|
|
Name the prefixes for the last names of covalent compounds that are used for the numbers 1 -10.
|
1 = mono
2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca |
|

Name the compound.
|
Nitrogen Monosulfide
|
|
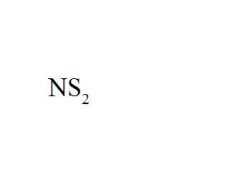
Name the compound.
|
Nitrogen Disulfide
|
|
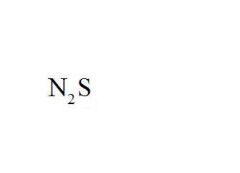
Name the compound.
|
Dinitrogen Monosulfide
|
|
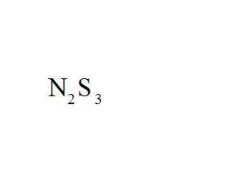
Name the compound.
|
Dinitrogen Trisulfide
|
|
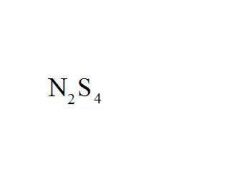
Name the compound.
|
Dinitrogen Tetrasulfide
|
|
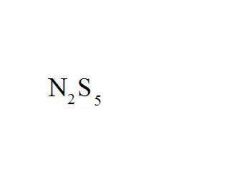
Name the compound.
|
Dinitrogen Pentasulfide
|
|
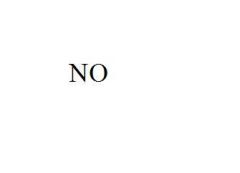
Name the compound.
|
Nitrogen Monoxide
Last syllable of the prefix (mono, di, tri, etc.) is dropped only with oxides |
|

Name the compound.
|
Nitrogen Dioxide
Last syllable of the prefix (mono, di, tri, etc.) is dropped only with oxides |
|
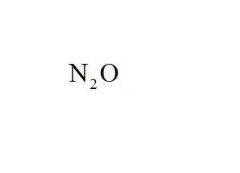
Name the compound.
|
Dinitrogen Monoxide
Last syllable of the prefix (mono, di, tri, etc.) is dropped only with oxides |
|

Name the compound.
|
Dinitrogen Trioxide
Last syllable of the prefix (mono, di, tri, etc.) is dropped only with oxides |
|

Name the compound.
|
Dinitrogen Tetroxide
Last syllable of the prefix (mono, di, tri, etc.) is dropped only with oxides |
|
|
Name the compound.
|

Dinitrogen Pentroxide
Last syllable of the prefix (mono, di, tri, etc.) is dropped only with oxides |
|
|
What is the chemical formula for water?
|
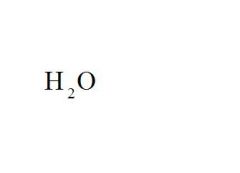
see the picture
|
|
|
What is the chemical formula for ammonia?
|
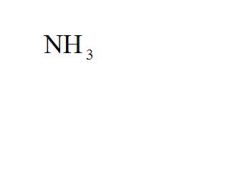
see the picture
|
|
|
What is the chemical formula for phosphine?
|

see the picture
|
|
|
What is the chemical formula for methane?
|
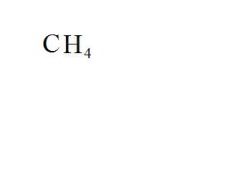
see the picture
|
|
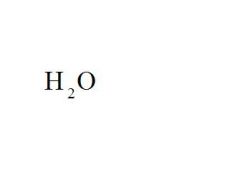
Name the compound.
|
Water
|
|
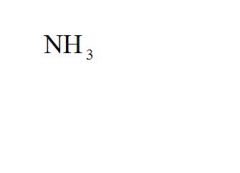
Name the compound.
|
Ammonia
|
|

Name the compound.
|
Phosphine
|
|
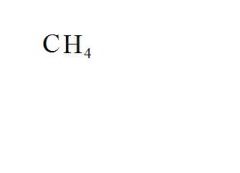
Name the compound.
|
Methane
|
|
|
_____ _____ _____ are the structures of covalent molecules or polyatomic ions that show the arrangements of the electrons around each atom.
|
Lewis Dot Structures
|
|
|
What is the Lewis Dot Structure of hydrogen?
|

see the picture
Note: The lone electron can be on any of the four sides of the hydrogen atom. |
|
|
Give the Lewis Dot Structure for oxygen.
|

see the picture
Note: Any two sides may have the single electrons, and any two sides can have the pairs. |
|
|
Give the Lewis Dot Structure for phosphorus?
|
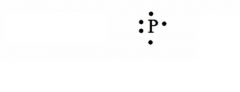
see the picture
Note: Any three sides can have single electrons, and any side can have a pair of electrons. |
|
|
Give the Lewis Dot Structure for chlorine.
|

see the picture
Note: Any three of the sides can have pairs of electrons, and any one side can have a single electron. |
|
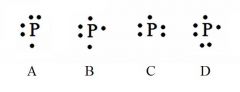
Decide whether the following are acceptable Lewis Dot Structures for phosphorus.
|
A - Incorrect. No side can have a second electron unless all sides have at least one.
B - Correct. C - Incorrect. No side can have a second electron unless all sides have at least one. D - Incorrect. Phosphorus has 5 valence electrons (not 6). |
|
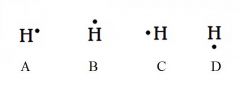
Decide whether the following are acceptable Lewis Dot Structures for hydrogen.
|
All are acceptable. Hydrogen has one electron, and it can be placed on any of the four sides.
|
|
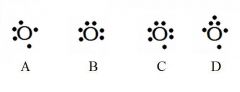
Decide whether the following are acceptable Lewis Dot Structures for oxygen.
|
A - Incorrect. Oxygen has 6 valence electrons (not 5).
B - Incorrect. No side can have a 2nd electron until all sides have at least one. C - Incorrect. Oxygen has 6 valence electrons (not 7). D - Incorrect. No side can have 3 electrons. |
|
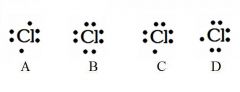
Decide whether the following are acceptable Lewis Dot Structures for chlorine.
|
A - Incorrect. Chlorine has 7 valence electrons (not 6).
B - Incorrect. Chlorine has 7 valence electrons (not 8). C - Correct D - Correct |
|
|
Name the six steps to determine how to make the Lewis Dot Structure for a compound?
|

see the picture
|

