![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
|
Atom |
the basic unit of a chemical element. |
|
|
Dalton |
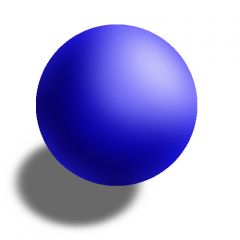
Thought that atoms look like a sphere that was the same throughout. |
|
|
Thomson |
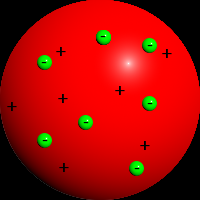
atoms must have an equal amount of positive and negative charge. |
|
|
Thomson |
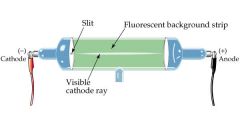
Cathode-Ray Tube experiment (CRT) |
|
|
Rutherford |
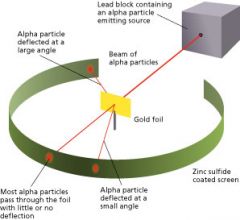
Gold Foil Experiment |
|
|
Rutherford |
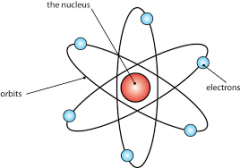
Dense, positively-charged mass in center of atoms. (nucleus) |
|
|
Electron |
Negative charge. Located in the electron cloud. |
|
|
Proton |
Positive charge. Located in the nucleus of an atom. |
|
|
Neutron |
Neutral charge. Located in the nucleus of an atom. |
|
|
Nucleus |
Located at the center of an atom. |
|
|
Atomic Number |
Number of protons in an atom of an element. |
|
|
Mass Number |
Sum of protons and neutrons in the nucleus of an atom. |
|
|
Isotopes |
Atoms of the same element that have different numbers of neutrons. |
|
|
Average Atomic Mass |
Average mass = (isotope mass x relative abundance) + ( isotope mass x relative abundance). |
|
|
Bohr |
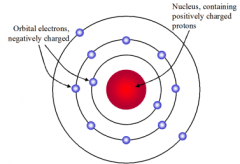
Electrons (e-) move with constant speed in fixed orbits around the nucleus, like planets around the Sun. |
|
|
De Broglie |
Moving particles like electrons have wave-like properties |
|
|
Heisenburg |
Heisenberg Uncertainty Principle- It is impossible to determine simultaneously both the location and the speed of an electron. |
|
|
Schrödinger |
Work lead to electron cloud model and later quantum model. |

