![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
68 Cards in this Set
- Front
- Back
|
Bronsted-Lowry Acid-Base Reactions |
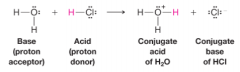
* Involves the transfer of protons |
|
|
Conjugate Acid |
* Molecule or ion formed when a base accepts a proton |
|
|
Conjugate Base |
Molecule or ion that forms when an acid loses its proton |
|
|
Brønsted-Lowry acid |
a substance that can donate (or lose) a proton. |
|
|
Brønsted-Lowry base |
* substance that can accept (or remove) a proton |
|
|
Strong Acids |
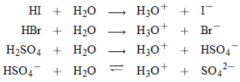
* Hydrogen Chloride (HCl) |
|
|
Diprotic Acid |
* Acid that can transfer 2 protons |
|
|
Hydronium Ion |
* strongest acid that can exist in water to any significant extent |
|
|
Solvated Ions |
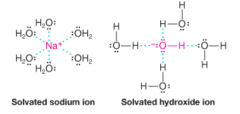
* When an ionic compound dissolves in water
|
|
|
Spectator Ion |
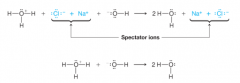
* play no part in the acid-base reaction
* Solvated ions |
|
|
Ionic Reaction |
* A reaction involving ions as reactants, intermediates, or products. Ionic reactions occur through the heterolysis of covalent bonds. |
|
|
Net Ionic Reaction when Solutions of all Aqueous Strong Acids and Bases are Mixed |
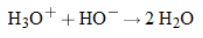
|
|
|
Curved Arrows |
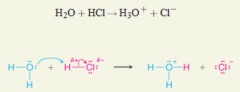
* Show the direction of electron flow in a reaction mechanism |
|
|
Lewis acid-base theory |
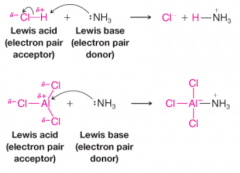
* Acids are electron pair acceptors
* Bases are electron pair donors |
|
|
Lewis Acid |
* Any electron deficient atom can act as a Lewis acid |
|
|
Lewis Acid-Base Reaction of Bromine and Ferric Bromide |
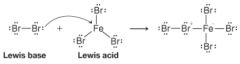
|
|
|
Lewis Acid-Base Reaction of Boron Triflouride and Ammonia |
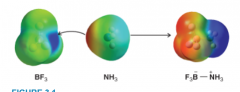
* BF3 has substantial positive charge on boron atom
* NH3 has negative charge located at nonbonding pair * Nonbonding electron fills boron's shell * Positive charge of product is localized near nitrogen * Negative charge of product is localized near boron trifluoride |
|
|
Heterolysis |
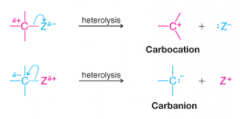
* Cleavage of a covalent bond so that one fragment departs with both of the electrons of the bond that joined them |
|
|
Carbocation |
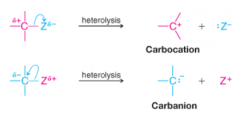
* Ion with positive charge on carbon atom
* Electron deficient (6 electrons in valence shell) * Lewis acid * Short lived and highly reactive * electrophile |
|
|
Carbanion |
* Ion with negative charge on carbon atom |
|
|
Electrophile |
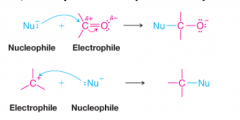
* Reagents that seek electrons as to achieve the stable shell configuration of a noble gas
* All Lewis acids are electrophiles * Atoms that are electron poor due to polarity are electrophiles |
|
|
Nucleophile |
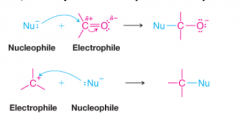
* Seeks a positive center |
|
|
Acid strength |
* The strength of an acid is related to its acidity constant, Ka or to its pKa. The larger the value of its Ka or the smaller the value of its pKa, the stronger is the acid. |
|
|
Acidity Constant (Ka) |
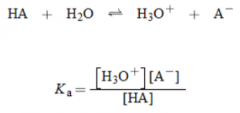
* If high (greater than 10), acid is a strong acid and if small, the acid is a weak acid
|
|
|
pKa |
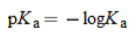
* Larger the value, weaker the acid
and the stronger the base |
|
|
pH |
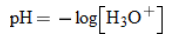
|
|
|
Predicting the Strength of Bases |
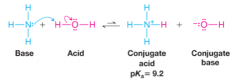
* The stronger the acid, the weaker its conjugate base |
|
|
Acid-Base Reactions |
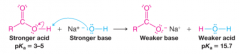
* Acid-base reactions always favor the formation of the weaker acid and the weaker base
* Increased difference of pKa of acids will more heavily favor the formation of products |
|
|
Carboxylic Acid Solubility as the Result of Salt Formation |
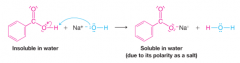
* Although acetic acid and other carboxylic acids containing fewer than five carbon atoms are soluble in water |
|
|
Amine Solubility as the Result of Salt Formation |
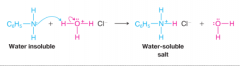
* Amines of low molecular weight are very soluble in water (methylamine)
* Amines of higher molecular weights like aniline are water-insoluable |
|
|
Strength of a Bronsted-Lowry Acid |
* Depends on extent to which a proton can be separated from it and transferred to a base |
|
|
Electronegativity and Acid Strength |

* Affects the polarity of the bond to the proton |
|
|
Hybridization and Acidity |

* having more s character means that the electrons of the anion will, on the average, be lower in energy, and the anion will be more stable
* Greater s character, means more acidity * An sp carbon atom is effectively more electronegative than an sp2 carbon, which in turn is more electronegative than an sp3 carbon * Opposite is true for base strength |
|
|
Inductive Effects |
* An intrinsic electron-attracting or -releasing effect that results from a nearby dipole in the molecule and that is transmitted through space and through the bonds of a molecule. |
|
|
Energy |
* Energy is the capacity to do work.
* release of heat results from a change from potential energy to kinetic energy in chemical reaction |
|
|
Kinetic Energy |
* Energy that results from the motion of an object.
|
|
|
Potential Energy |
* Stored Energy |
|
|
Relative Stability |
* The more potential energy an object has, the less stable it is |
|
|
Potential Energy and Covalent Bonds |
* release of heat results from a change from potential energy to kinetic energy |
|
|
Enthalpy Change |
* measure of the difference in the total bond energy of the reactants and products. |
|
|
Exothermic Reactions |
* Negative change in enthalpy
* Evolve heat |
|
|
Endothermic Reactions |
* Positive change in enthalpy |
|
|
Free Energy Change |
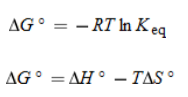
* Reaction is favored if negative (equilibrium greater than 1) |
|
|
Acetic Acid vs. Ethanol Acidity |
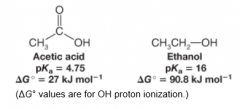
* Ethanol is less acidic than acetic acid since it is less favorable to lose an H |
|
|
Stability of Acetate Ion vs. Ethoxide Ion |
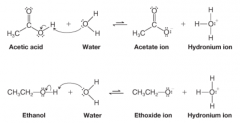
* Delocalization of charge (as depicted by reasonance structures of carboxylate ion) |
|
|
Delocalization Effect (by reasonance) |
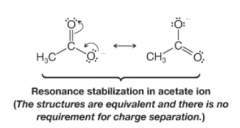
* Negative or positive charge is distributed evenly stabilizing charge
|
|
|
Inductive Effect - Acetic Acid & Ethanol |
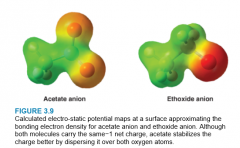
* Acetate anion's electron-withdrawing effect is distributed amongst O atoms due to reasonance
* Ethanol's anion's electron-withdrawing effect is localized to only 1 O atom * Acetate Anion is more stable |
|
|
Substituent Effect |
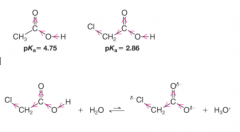
* greater acidity of chloroacetic acid can be attributed, in part, to the extra electron-attracting inductive effect of the electronegative chlorine atom |
|
|
Halogen Elements |
Fluorine (F); Chlorine (Cl); Bromine (Br); Iodine (I); Astatine (At) |
|
|
Acetone |
- simplest Ketone - (CH3)2CO |
|
|
Acetaldehyde |
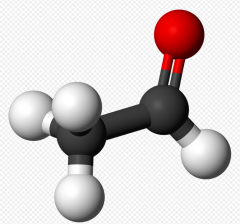
- CH3CHO |
|
|
Protic Solvent |
* Contains a H atom attached to a strongly electronegative element such as O or N |
|
|
Acidity in the Gas State |
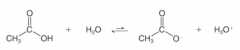
* In the absence of a solvent (i.e. in the gas phase) most acids are weaker than in a solution |
|
|
The Effect of the Solvent on Acidity |
* The stability of a conjugate base is enhanced if it is solvated to a greater extent than the corresponding acid. |
|
|
Pronated alcohol |
* Conjugate acid of the alcohol |
|
|
Alkyloxonium Ion |
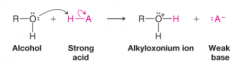
An alcohol with an oxygen with a formal positive charge |
|
|
Dialkyloxonium Ion |
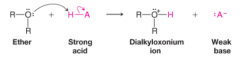
* An ether containing an O with a positive formal charge
|
|
|
Protonated Ketone |
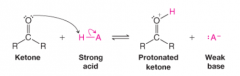
* Compounds containing a carbonyl group also act as bases in the presence of strong acid |
|
|
Substitution Reaction |
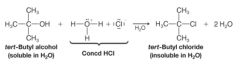
* A reaction in which one group replaces another in a molecule.
|
|
|
Reaction of tert-Butyl Alcohol with Concentrated Aqueous HCl |
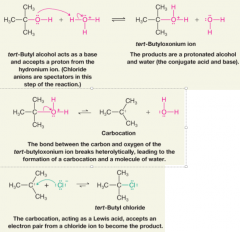
* Step 2 occurs because alcohol is protonated, positive charge on O weakens C-O bond
* why doesn't a molecule of water (also a Lewis base) instead of a chloride ion react with the carbocation in step 3? (3.12) |
|
|
Leveling Effect of Solvent |

* An effect that restricts the use of certain solvents with strong acids and bases.
* no acid stronger than the conjugate acid of a particular solvent can exist to an appreciable extent in that solvent * no base stronger than the conjugate base of the solvent can exist to an appreciable extent in that solvent. |
|
|
Alcohols as Solvents |
* Often used for organic reactions
* Less polar than water, so dissolve less polar compounds * Offers advantage of using alkoxide ions (RO-) as bases (stronger base than hydroxide ions) |
|
|
Alkyllithium |
* RLi
* Although has covalent character, it is polarized to make C negative * Reacts as though it contains alkanide ions |
|
|
Alkanide ions |

* Conjugate bases of alkanes
* Strongest bases |
|
|
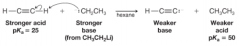
Terminal Alkynes |
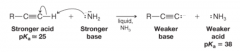
* alkynes with a proton attached to a triply bonded carbon
* Have a pKa of about 25 * React with sodium amide in liquid ammonia |
|
|
Deuterium |
* Isotope of hydrogen
* 2 amu |
|
|
Tritium |
* Isotope of hydrogen
* 3 amu |
|
|
Duetrium and Tritium Labeled Compounds |

* Duetrium or tritium replace 1 or more H atoms of a compound |

