![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
Q>k |
Too much products Shifts toward reactants |
|
|
Q<k |
Too much reactants Shifts toward products |
|
|
Buffers |
Closest pka to ph is when the buffer works best Buffers work best when the base and acid concentration is about the same |
|
|
H-H |
ph=pka+log(base\acid) |
|
|
Lower concentration |
Higher ph |
|
|
Larger ka |
Stronger acid |
|
|
Ph>pka h-h |
More base |
|
|
Ph<pka h-h |
More acid |
|
|
Only for conjugates |
Kw=ka×kb Where kw = 1×10^-14 |
|
|
SA/SB |

|
|
|
WA/SB |
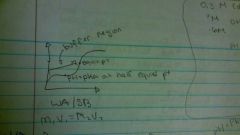
|
|
|
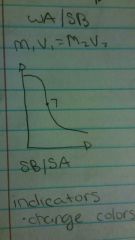
SB/SA |
|
|
|
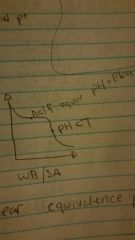
WB/SA |
|
|
|
Half equivalence point |
Ph=pka |
|
|
Ksp |
[Ions] |
|
|
Ksp=x^2 |
2 ions |
|
|
Ksp=4x^3 |
3 ions |
|
|
Ksp=27x^4 |
4 ions |
|
|
Ph=14-poh |
. |
|
|
Kb |
Same as ka but whem you do -log you find the poh not ph value then you subtract from 14 to find ph |

