![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
22 Cards in this Set
- Front
- Back
|
structural isomers
|
molecules with same molecular formula but different structures.
|
|
|
Stereoisomers
|
molecules in which the same connectivity but a different spatial arrangement. Can be geometric or optical isomers.
|
|
|
optical isomers
|
Two molecules that are nonsuperimposable mirror images of one another, ex left and right hand.
|
|
|
chiral
|
to be an optical isomer (a.k.a an enantiomer) an enantiomer is chiral. they differ in the direction in which they rotate polarized light and in their chemical behavior in a chiral environment.
|
|
|
dextrorotatory isomer
|
the enantiomer that rotates the polarization of the light clockwise
|
|
|
levorotatory isomer
|
the enantiomer that rotates the polarization of the light counterclockwise
|
|
|
racemic mixture
|
an equimolar mixture of both optical isomers that do not rotate the polarization of light at all.
|
|
|
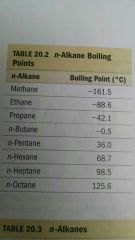
the boiling point of alkanes...?
|
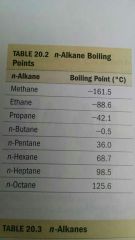
increases with increasing carbon.
|
|
|
hydrocarbon uses and states
|

|
|
|
alkyl groups
|
branch off main chain, named with prefix-yl
|
|
|
naming alkanes
|
chain#,#-substituentbase
|
|
|
cis isomer
|

same side (top or bottom)
|
|
|
trans isomer
|

across.
|
|
|
stereo isomer
|
same form same bond diff arrangement cis and trans
|
|
|
benzene prefixes
|
1,4 para. 1,3 meta 1,2. ortho
|
|
|
alcohols
|
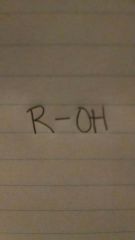
-anol
|
|
|
ethers
|
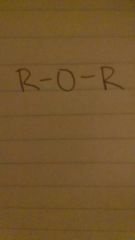
ether
|
|
|
aldehydes
|
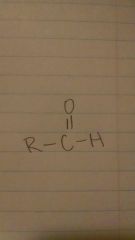
-anal
|
|
|
ketones
|
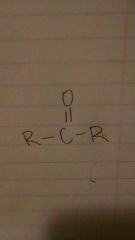
-anone
|
|
|
carboxylic acids
|
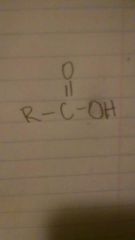
-oic acid
|
|
|
esters
|
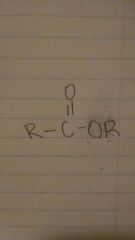
acetate
|
|
|
amines
|
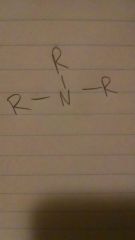
-amine
|

