![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
153 Cards in this Set
- Front
- Back
|
What is induction?
|
Any mechanism whereby one population of cells influences the development of neighboring cells.
|
|
|
What is meant by threshold in terms of development?
|
The concentration at which a signal induces a specific cellular response. Often, the inductive signal induces an entire tissue containing multiple cell types.
|
|
|
What is mean by competence?
|
Cells that are able to respond to induction signals are said to be competent. Cells differ in their ability to respond to inducing signals.
|
|
|
What are the factors that can determine competence?
|
1. Express particular signaling molecule.
2. Able to activate specific intracellular signaling pathways. 3. Presence of the transcription factors required to stimulate expression of the genes required to implement the developmental pathway induced. |
|
|
What is an example of a group of related extracellular signaling molecules that regulate development?
|
Transforming Growth Factor β (TGF β)
|
|
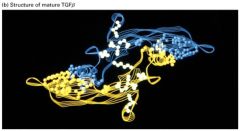
Describe this structure.
|
1. The monomers have 6 cysteine residues which form 3 intrachain disulfide bonds.
2. This cysteine knot domain is relatively resistant to denaturation and thought to be particularly important for extracellular induction molecules. 3. Monomers form dimers by interchain disulfide bonds forming b/w additional N-terminal cysteins in monomers. These proteins can be homo- or heterodimetic. |
|
|
What are 3 main types of TGFβ receptor proteins?
|
Type I/II
: Transmembrane serine/threonin kinases ->activat its kinase activity Type III : Cell surfae proteoglycan (β-glycan); regulates accessibility of TGFβ to Type I/II receptors. |
|
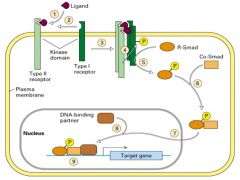
How are the TGFβ dimers and receptors involved in developmental pathways?
|
1. TGFβ superfamily of proteins are extracellular signaling molecules with widespread roles in regulating growth and development (dorsoventral cell fates).
2. A general model of the TGFβ signaling pathway begins with a TGFβ dimer binding to type II TGFβ receptors at the cell surface. 3. TGFβ dimer induces formation of a complex b/w type II and I receptors. 4. Once bound to TGFβ, a type II receptor phsphorylates and activates a type I receptor. 5.Smad proteins transduce the signal downstream of TGFβ receptors. The activated type I receptor phosphorylates with a receptor-regulated Smad (R-Smad), which then dimerizes with a co-Smad. 6. THe Smad dimer moves into the nucleus and with a DNA-binding partner (molecule), activates transcription of target genes. |
|
|
Who are the 3 members in TGFβ Superfamily?
|
Dpp, BMP, TGFβ
|
|
|
What is Dpp gene?
|
TGFβ superfamily found in vertebrates.
Acts as a morphogen to induce establishment of different ectodermal cell types at different threshold concentrations during development. More Dpp -> more dorsal cells; other proteins (such as tolloid or sod) may control Dpp activity |
|
|
What is BMP gene?
|
TGFβ superfamily found in frog.
It's similar role to Dpp, but it's controlled by chordin instead of tolloid and sog. |
|
|
What is the effect of Dpp protein on dorsoventral patterning in vertebrate embryo?
|
It establishes ectodermal cell tyepes-> dorsal structures.
Its concentration is greatest in the most dorsal (top) region and when it's absent, the cells develop ventral neurogenic tissues. |
|
|
What's the advantages of using xenopus in developmental biology research?
|
Large-size embryo
Easy to experimentally manipulate |
|
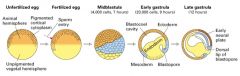
Explain how the sequential inductive events that regulate early xenopus development.
|
1. Unfertilized egg divided into 2 broad hemispheres giving it an intrinsic symmetry.
2. Site of sperm entry defines the ventral side of the embryo and leads to rotation of the cortical cytoplasm. 3. Fertilization -> rapid cell divisions and formation of the early blastula (hollow ball of 32 cells called blastomeres). 4. Signals from the vegetal pole induce formation of mesodermal cells in the marginal zone (blue) separating the vegetal and animal ends in the midbalstula stage. 5. During gastrulation, mesodermal cells fold into the embryo. 6. Signals from the invaginating mesoderm induce the development of both the underlying ectoderm and neuronal tissue from the overlying ectoderm. 7. Anteriposterior axis is determined by the mesoderm; cells that invaginate first induce anterior structures. 8. Subsequent interactions b/w different cell populations play an important role in organogenesis. |
|
Explain the induction in Xenopus embryogenesis.
|
1. Early in vertebrate embryogenesis, the 3 germ layers - ectoderm, mesoderm, and endoderm - are established.
2. During the subsequent gastrula stage, the mesoderm induces the overlying ectoderm to form neuronal tissue and the endoderm to form tissues such as the pharynx and intestine. |
|
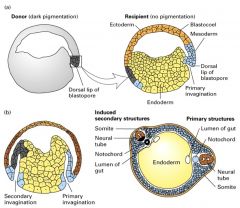
Explain how the sequential inductive events that regulate early xenopus development.
|
1. Unfertilized egg divided into 2 broad hemispheres giving it an intrinsic symmetry.
2. Site of sperm entry defines the ventral side of the embryo and leads to rotation of the cortical cytoplasm. 3. Fertilization -> rapid cell divisions and formation of the early blastula (hollow ball of 32 cells called blastomeres). 4. Signals from the vegetal pole induce formation of mesodermal cells in the marginal zone (blue) separating the vegetal and animal ends in the midbalstula stage. 5. During gastrulation, mesodermal cells fold into the embryo. 6. Signals from the invaginating mesoderm induce the development of both the underlying ectoderm and neuronal tissue from the overlying ectoderm. 7. Anteriposterior axis is determined by the mesoderm; cells that invaginate first induce anterior structures. 8. Subsequent interactions b/w different cell populations play an important role in organogenesis. |
|
|
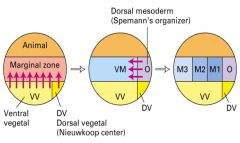
What is the function of the Spemann organizer (dorsal lip)?
|
1. Induces neuronal tissue from ectodermal tissues.
2. It dorsalizes the mesoderm. |
|
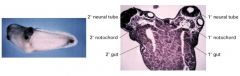
What molecules in the dorsal lip of vertebrate gastrulas induce twinned embryos?
|
Chordin recruited host tissue to form the twin in xenopus
|
|
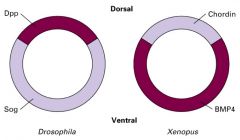
Explain how TGFβ homologs have revers expression pattern.
|
Chordin's protein structure in Xenopus is very similar to the Sog protein in Drospophila. SOg protein plays a role in developing ventral cells, tissues and structure (opposite effect to dpp protein in Drosophila).
Chordin in frogs plays role in developing dorsal cells, tissues and structure. In vertebrates the nervous system forms dorsally and in invertebrates, it forms ventrally. |
|
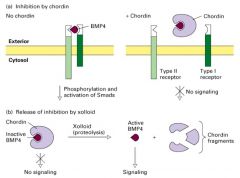
How is BMP signaling Regulated?
|
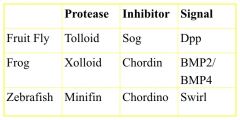
By the binding of chordin which keeps BNP from binding to the Type II receptor; thus phosphorylation and activation of Smads and ultimately transcription of the target gene does not occur.
Xooloid (tolloid in Drosophila) specifically cleaves (proteolysis) chordin (Dpp in drosophila) in the chordin BMP complex releasing BMP in a form that can bind to its receptor and trigger TGFβ signaling. |
|
|
What is the difference between apoptosis and necrosis?
|
Necrosis is the process in which cells die in response to tissue damage. These cells swell and burst, releasing their intracellular contents, which can damage surrounding cells and often causes inflammation.
|
|
|
What is programmed cell death?
|
A central mechanism in controlling multicellular development.
Leads to the deletion of entire structures. Regulates the number of neurons in the nervous system In the mammalian nervous system, the majority of cells generated during development also die during development. |
|
|
What is trophic factor?
|
It's a survival signal.
W/o TF, the self-destruction program will be activated. |
|
|
What does programmed cell death occur through apoptosis?
|
Dying cells shrink, and condense, then fragment, releasing small membrane-bound apopotic bodies, which are generally phagocytose by other cells.
Intracellular components are not released extracellularly where they might have negative effects on neighboring cells. |
|
|
What are the morphologic changes in apoptopic cells?
|
Dense chromosome condensation occurs along the nuclear periphery. The cell body shrinks, although most organelles remain intact. The nucleus and cytoplasm fragment, forming apoptoic bodies. These are phagocytosed by surrounding cells.
|
|
|
What are the examples of neutrophins?
|
Nerve Growth Factor (NGF), Brain Derived Growth Factor (BDGF) and neutrophin-3 (NT-3)
|
|
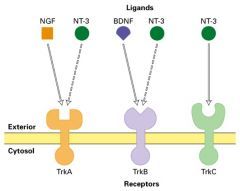
What is a neutrophin?
|
Neutrophin bind to and activate a family of receptor tyrosine kinases called Trks ; this binding provides a survival signal for different classes of neurons.
|
|
|
How are the effects of neutrophins and receptors on neurons in mice differ?
|
TrkA and NGF -> pain sensitive nociceptive neurons
TrkC and NT3 -> detect position of the limbs; proprioceptive neurons TrkB and BDNF -> development of sensory neurons in the vestibular ganglia. (sensing motion) |
|

Explain Knockout Mice Experiment
|
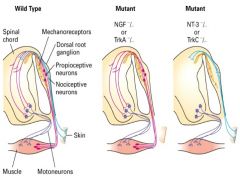
Mice with knockout mutations in each of the neurotrophins and their receptors were produced in the lab.
Pain-sensitive (nociceptive) neurons which express TrkA are selectively lost in the dorsal root ganglion of knockout mice lacking NGF or TrkA, where as Trk-B and Trk-C expression neurons are unaffected by such knockouts. Trk-C expressin proprioceptive neurons, which detect the position of the limbs, are missing from the dorsal root ganglion in TrkC and NT3 mutants. In BDNF mutants, animals show defects in balance associated with the vestibular system. |
|
What are 3 classes of proteins function in the apoptotic pathway in vertebrates?
|
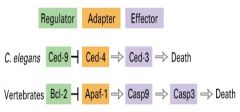
1. Regulators
- family of proteins that can either promote or suppress apoptosis. 2. Adapters - Interact with regulators and effectors; in the absence of trophic factors, they promote activation of effectors. 3. Effectors - Family of cysteine proteases; their activation leads to degradation of various intracellular substrates and eventually cell death. |
|
|
What is the role of pro-apoptotic regulators?
|
It promotes Caspase activation.
|
|
|
What are Capsases?
|
They are a family of enzymes that are the effector molecules in the apoptotic pathway. It's called capsases because they are cystein proteases that selectively cleave proteins at sites just C-terminal to aspartate residues.
|
|
|
What are the examples of pro-apoptotic regulators?
|
- all members of the regulators protein family are referred to as the Bcl-2 family; even though Bcl-2 suppresses apoptosis.
ex) Bax Bcl-2 family members can influence the subcellular distribution of cytochrome c; and cytochrome c is involved in capases activation. |
|
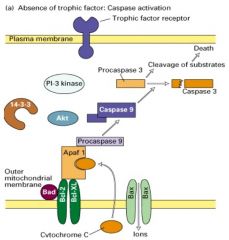
Explain apoptosis pathway.
|
1. in the absence of a trophic factor, Bad (a soluble pro-apoptotic protein) binds to the anti-apoptotic proteins Bcl-2 and Bcl-xl, which are inserted into the mitochondrial membrane.
2. Bad binding prevents the anti-apoptotic proteins from interacting with Bax, a membrane-bound pro-apoptotic protein. 3. Bax forms homo-oligomeric channels in the membrane that mediate ion flux. 4. This leads to the release of cytochrome c from the space between the inner and outer mitochondrial membrane. 5. Cytochrome c them binds to the adapter protein Apaf-1, which in turn promotes a capsase cascade leading to cell death. |
|
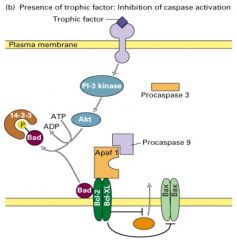
Explain Cell-survival pathway.
|
1. In the presence of a trophic factor such as NGF, some cells stimulate PI-3 kinase activity
2. Leading to activation of the downstream kinase Akt, which is phosphorylates Bad 3. Phosphorylated Bad then forms a complex with the 14-3-3 protein. 4. With Bad sequestered in the cytosol, the antiapoptotic Bcl-2/Bcl-xl proteins can inhibit the activity of Bax, thereby preventing the release of cytochrome c and the activation of the caspase cascade and the cell survives. |
|
|
What are various integral membrane proteins called?
|
Cell-adhesion molecules (CAMs)
|
|
|
What is the function of the extracellular matrix (ECM)?
|
1. Helps bind the cells in tissues together
2. Reservoir for many hormones controlling cell growth and differencitation 3. Provides a lattice through which cells can move 4. Defects in thsese connections lead to cancer and developmental malformations. |
|
|
What are various integral membrane proteins called?
|
Cell-adhesion molecules (CAMs)
|
|
|
What is the function of the extracellular matrix (ECM)?
|
1. Helps bind the cells in tissues together
2. Reservoir for many hormones controlling cell growth and differencitation 3. Provides a lattice through which cells can move 4. Defects in thsese connections lead to cancer and developmental malformations. |
|
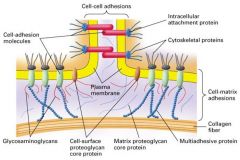
3 major protein components of the ECM?
|
1. Proteoglyans
- cushion cells 2. collagent fibers - provide strength and resilience 3. multiadhesive matrix proteins - bind to receptors on the cell surface |
|
|
Explain characteristics of cell adhesion molecules (CAMs)
|
1. Cell-surface proteins
2. help cells of like type to bind together 3. Most CAMs are uniformly distribuited along the regions of the plasma membrane that contact other cells 4. cytosol-facing domains of these proteins are usually connected to elements of the cytoskeleton |
|
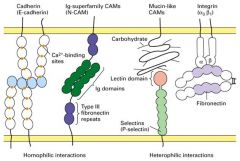
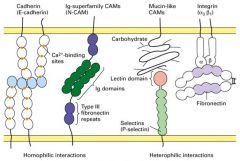
What are the 5 principal classes of CAMs?
|
1. Cadherins
2. Ig Superfamily (N-CAMs) 3. Selectins 4. Mucins 5. Integrins |
|
|
What are the 4 major classes of cell junctions?
|
1. Tight junction
- connect epithelial cells 2. Gap junction -distributed among adjacent cells allow the exchange of small molecules 3. cell-cell junction 4. cell-matrix junction |
|
|
What are the roles of Cadherins and integrins?
|
1. Used in cell-cell and gap junctions
2. Assist in connecting the internal cytoskelton directly to the cell exterior or the extracellular matrix |
|
|
What are the 3 parts that all cytoskektal junctions have?
|
1. Cell adhesion molecules (CAMs)
- connect the cell to another cell or the ECM 2. Adapter proteins - connect the CAMs to action or keratin filaments 3. Bundle of cytosekeltal filaments. |
|
|
What is Cadherins?
|
1. Ca2+ dependent.
2. Major molecules of cell-cell adhesion (homophillic). 3. Play a critical role in tissue differentiation. 4. there are over 40 different types of cadherins 5. The brain expresses the largest number of different cadherins 6. Metastasis of tumor cells is correlated with loss of cadherin on their cell surface. |
|
|
How do we know that E-cadherin is required for cell-cell adhesion in epithelial sheets?
|
when an antibody to E-cadherin is added to cultured epithelial cells, the cells detach from each other.
if Ca2+ is removed, it will disrupt |
|
|
What is the characteristics of N-CAMs?
|
1. Ca2+ independent
2. Homophiilic (bind together cells with similar N-CAMs) 3. Play an important role in differentiation of muscle, glial, and nerve cells 4. Adhesion of cultured retinal neurons in inhibited by addition of antibodies to N-CAMs. 5. Encoded by a single gene; diversity comes from alternative mRNA splicing and differences in glycosylation. |
|
|
What is the effect of sialic acid of N-CAMs?
|
1. Sialic acid forms weaker homophillic interactions due to repulsion b/w negatively charged acid residues.
2. Embryonic tissue has 25% polysialic acid and it's good for nervous system. 3.Adult tissues contain only ~7.5% polysialic acid to stabilize cell contacts. 4. The strength of cell-cell adhesions is modified during differentiation by differential glycosylation of the N-CAMs. |
|
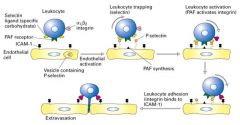
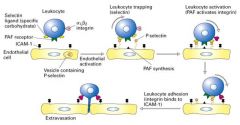
Explain extravasation.
|
1. leukocyte normally circulate in the blood unattached.
2. The presence of an infection or inflmmation triggers leukycytes to move into tissue toward the foreign invader. 3. First, inflmmatory signals (cytokine) induce endothelial cells to exocytose P-selectin and synthesize platelet activating factor (PAF), which appear on the endothelial cell surface. 4. P-selectin molecules bind oligosaccharide groups at the leukocyte cell suface. Since this adhesion is weak, the cell rolls along the endothelial cells. 5. Leukocyte contains PAF receptors and integrins. Receptor binding the PAF results in the activation of the integrins. 6. The activated integrins are able to bind ICAM (Ig superfamily(. This causes the leukycyte to stop rolling and to tightly adhere to the endothelium. 7. The adhered leukocyte then begins its migration to reach the underlying tissue. |
|
|
Why is Beta2 subunit found in integrin significant?
Humans with a genetic defect in synthesis of integrine beta2 leads to what type of disease? |
Beta2 subunits are found in integrins and are involved in cell-cell adhesion.
Humans -defect in synthesis of integtrin beta2 subunit have leukycyte adhesion deficiency and are susceptible to repeated bacterial infections. |
|
|
What were the 3 components to cell-cell connections again?
|
1. Cell adhesion molecules (CAMs) - connect the cell to the other cell or the ECM
2. Adapter proteins 3. Bundle of cytoskeltal filaments |
|
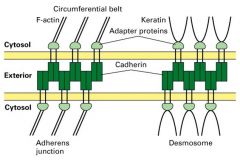
Explain cell-cell adhesion molecues in junctions.
|
M
|
|
|
What are the examples of cadherin cell adhesion molecules?
|
desmoCOLLIN
desmoGLEIN |
|
|
How was desmoglein first identified?
|
Through the autoimmune skin diease called pemphigus vulgaris.
The patiens made antibodies that bind to a normal body protein. Therefore, autoantibodies disrupted adhesion b/w epithelial cells causing blisters of the skin and mucous membranes. |
|
|
What is desmosomes?
|
Components b/w epithelial cells and attachments to the sides of keratin intermediate filaments.
|
|
|
What functions as gap junction in plants?
|
Plamsa desmata
|
|
|
Explain the cylindrical particles in gap junctions
|
they're water filled channels that directly link the cytosol of one cell to another, providing passageway for very small molecules (such as AA, nucleoside phosphate, cAMP)
and ions b/w the cells. |
|
|
What is the significance of cAMP and Ca2+ being able to move across gap junctions?
|
1. cAMP and Ca2+ are second messengers.
2. The hormonal stimulation of just 1 or a few cells in an epithelium initiates a metabolic reaction in many of them. 3. Gap junction-mediated transfer of Ca2+ ions b/w adjacent smooth muscle cells allows the coordinating contractile waves in the intestine during peristalsis and the uterus during birth, digestion.. etc. |
|
|
What are the structures of gap junctions?
|
1, Connexon subunit
- hexagonal particle with a hollow core - contain 12 connexin molecules (proteins)' 6 in each cell as hexagonal hemichannels 2. Connexin subunit - each connexin protein spans the plasma membrane 4 times; and an alpha-helix lines the aqueous channel. |
|
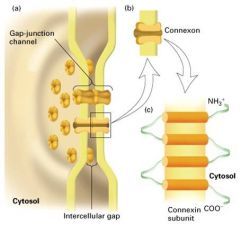
Explain the structure of gap junctions?
|
- the connexin proteins differ in their C-terminal segment which faces the cytosol.
- there are several types of connexin (at least 12) - some cells express a single connexin, their channels are homotypic, consisting of identical connexons - most cells express at least 2 connexin genes -> heterotypic channels - the diversity in channel composition leads to differences in permeability of channels to different molecules. |
|
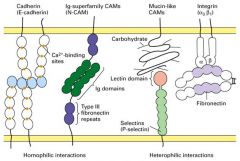
Explain integrin.
|
1. heterodimers of alpha and beta subunits
2.the ligand-binding site is composed of parts of both chains. 3. In mammals, at least 22 integrin heterodimers are known that bind to different ligands. 4. cell-matrix adhesions are involved in embryogenesis 5. integrins are gouped in subfamilies sharing a common Beta subunit |
|
What kinds of interaction do integrins have?
|
1. Integrins have low affinities for their ligands.
2. but since hundreds or thoussand of integrin molecules are bnding to extracellular matrix proteins, the cell remains firmly anchored to the extracellular matrix. 3. since interactions are weak, cells are able to migrate and "fine-tune" their interaction with the matrix. |
|
|
How is cell-matrix adhesion modulated?
|
Example:
Platelet activation involves a comformational changes in the specific alph beta integrin. Attachment of cells to matrix components can be modulated by down-regulating the number of integrin molecules on the cell surface. |
|
|
What type of De-adhesion factors promote cell migration?
|
Disintegrins (de-adhesion factors)
- bind to integrins on the surface of cells and competitively inhibit binding of cells to matrix components - one class is present in snake venoms that prevent platelets from aggregating (promote cell migration) - another class contains 2 types of proteases, fibrinogen, and matrix-specific metalloproteinases (NMPs). Both proteases degrade matrix components, therby permitting cell migration. |
|
|
Cell surface remoel is abbreviated as ADAM? what does it stand for?
|
A Disintegrin And a Metallopretease domain.
- It's a family of membrane-cnchored glycoproteins. |
|
|
Explain ADAM.
|
- Involved in a variety of events that depend on remodeling the cell surface.
- Determination of cell fates during embryogenesis - Fusion of a sperm and egg during fertilization - Release of soluble tumor necrosis factor alpha (TNF alpha) - Involved in "ectodomain shedding" -> permits the cell to inactivate membrane receptors or release soluble active proteins from the cell surface. -all new cell adhesion |
|
|
What are the exterior structures in integrin-containing junctions?
|
1. Focal adhesions
- attach the actin cytoskeleton to fibers of fibronectin (multi-adhesive matrix protein) 2. Hemidesmosomes - connect intermediate filaments to basal laminae (laminin, a multi-adhesive matrix protein) |
|
|
What are some facts of collagen?
|
1. The major insoluble finbrous protein in the extracellular matrix and in connective tissue
2. The single most abundant protein in the animal kingdom. 3. There are at least 16 types of collagen; but 80-90% of the collagen in the body consists of types I,II,III |
|
|
What are 3 main types of polymerized structures of collagen?
|
1. Fibrillar collagens
- skin, tendon, bone 2. Fibril-associated collagens - most intestital tissues, cartilage, vitreous humor 3. Sheet-forming collagents - all basal laminates |
|
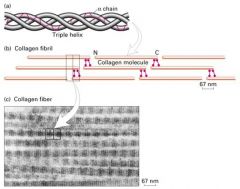
What is the structure of Type I collagen?
|
Long thin protein made of 3 coiled subunits
- 2 alpha1 chain and 1 alpha2 chains. -each chain contains 1050 amino acids wound around each other in a right-handed triple helix - stabilized by covalent bonds b/w the N-terminus of one molecule and C-terminus of an adjacent one. -type I fibrils are packed sided-by-side in parallel bundles, called collagen fibers |
|
|
How does collagen get its triple helical structure?
|
1. From unusual abundance of glycine, proline, and hydroxyproline.
2. Repeating motif would be: Gly-Pro-X (X=any AA): there is a glycine at every third position for the collagen triple helix to form. |
|
|
What are the structures of collagen?
|
Triplestranded helical molecule.
- in fibrous collagen, molecules pack together side by side. - Adjacent molecules are displace slightly such that a small gap separates the "head" of one collagen from the "tail" of the next. - This side to side interactions are stabilize by covalent bonds. |
|
|
Wher does the assembly of collagen fibers begin and completed??
|
Begins in the rough ER and completed outside the cell.
|
|
|
What are procollagens where is it found?
|
Synthesizing collegen chains as longer precursors. It's found on robosomes of the rough ER.
|
|
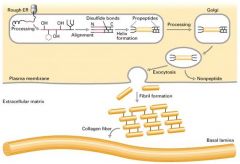
Explain the process of procollagen becoming a collagen fiber.
|
1. Growing peptide chains are transported into the lumen of the rough ER.
2. in the ER, the procollagen chain undergoes a series of processing reactions, including hydroxylation, glycosylation, and disulfide-bond formation. 3. Disulfide bonds from b/w N and C-terminal polypeptides 4. Procollagen is secreted into the extracellular space where it is polymerized into fibrils. 5. Propeptides (at the ends) are removed by enzymes. |
|
|
How does the disease scurvy relate to collagen formation?
|
-When vit C (ascrobic acid) is missing, procollagen chains are not hydroxylated sufficiently to form stable triple helices at normal body temperatures (Collagen can't be processed normally)
- Procollagens can'tform normal fibrils - nonhydroxylated procollagen chains are degraded within the cell -w/o the structural support of collagen, blood vessels, tendon, and skin become fragile. |
|
|
GIve examples of Collagen mutations.
|
1. Type I collagen fibrils are used as the reinforcing rods in construction of bone.
2. Certain mutations in the alpha1 or alpha2 genes lead to osteogenesis imperfecta (brittle-bone disease) 3. Most severe form is an autosomal dominant, lethal disease resulting in rapid death either in utero or shortly after birth. 4. Fibrous colalge is very susceptible to mutation, especially in glycine residues. 5. Because mutant collagen chains can affect the function of wild-type ones, such mutations have a dominant phenotype. |
|
|
What are other classes of proteins beside collage in the ECM?
|
1. Multiadhesive matrix proteins
2. Proteoglycans |
|
|
What connects multiadhesitv matrix protein of the ECM?
|
Integrins (one of the CAMs)
|
|
|
What are some examples of multi-adhesive matrix proteins?
|
1. Laminin
2. Fibronectin 3. Entactin |
|
|
What are some examples of proteoglycans?
|
Aggregan (ECM)
Syndecan (cell surface) Perlecan (basal lamina) |
|
|
WHat is an important polysaccharide component of the ECM?
|
Hyaluronan:
A large polysaccharide that form a highly hydrated gel, making the ECM resilient to compression. |
|
|
What are some characteristics of multiadhesive matrix proteins?
|
1. Long, flexible molecules contain domains for binding a variety of collagens, other matrix proteins, polysaccarides, cell-surface proteins, and signaling molecules such as growth factors and hormones.
2. Major role is to attach cells to the extracellular matrix 3. Important in initiating cellular responses through signal trasduction pathways. 4. Organize other components of the matrix and regulating cell attachment to the matrix, cell migration and cell shape ------------------------------------- To sum up, MMP is STICKY! |
|
|
Explain proteoglycans.
|
1. Found in all connective tissues and ECMs.
2. Contain a high content of polysaccharides and are thus highly hydrated. 3. The swelled, hydrated structure of proteoglycans -> the volume of the ECM and also acts to permit diffusion of small molecules b/w cells and tissues. |
|
|
What is basal lamina?
|
Thin sheet-like network of ECm components that underlies most animal epithelial layers and other organizing groups of cells, separating them from connective tissue.
Laminin and Type IV colalgen form the 2-D reticulum of the basal lamina. All the ECM components are made by cells that rest on the basal lamina. |
|
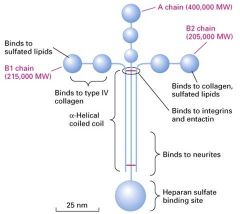
What type of protein is this?
|
Laminins:
1. family of cross-shaped proteins as long as the basal lamina is think 2. a heterotrimeric protein in adult animals 3. Contains A,B, or C chains 4. Contains a coiled-coil region in which the 3 chains are covalently linked via several disulfide bonds. 5. Different regions (domains) of laminin bind to cell-surface receptors and various matrix components |
|
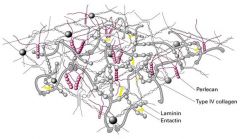
What are the structures shown in the diagram?
|
1. Perlecan
- a heparan sulfate proteoglycan 2. Entactin - A small multiadhesive matrix protein that interacts with laminin and type IV collagen |
|
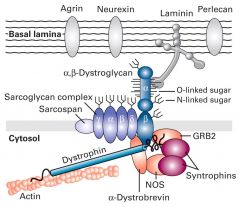
what is Dystroglycan and Dystrophin glycoprotein complex (DGC)?
|
- large glycoprotein precursor, proteolytically cleaved into 2 subunits; alpha is a peripheral membrane protein and beta is a transmembrane protein that associates with beta.
-O-linked sugars are covalently attached to hydroxyl groups of serine and threonin residues in the alpha subunit - these O-linked sugars bind to basal laminal component; (multi-adhesive proteins: laminin, and the proteoglycans perlecan and agrin). - The beta segment binds further to dystrophin and other adapter proteins and intracellular signaling proteins. |
|
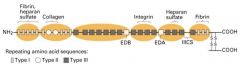
What is the structure of fibronectin chains?
|
1. Dimers of 2 similar polypeptides linked at their C-termini by 2 disulfide bonds.
2. At least 20 different fibronectinc chains have been identified 3. Polypeptides consist of 6 domains 4. Each domain contains repeated sequences beloning to one of 3 types (I,II, III) |
|
|
What's the role of fibronectin?
|
1. Multiadhesive matrix proteins.
2. Attach cells to all matrixes that contain the fibour collagens. 3. Regulate the shape of cells and the organization of the cytoskelton 4. Important in wound healing |
|
|
What is glycosaminoglycans (GAGs)?
|
Long repeating linear polymers of specific disaccharides in proteoglycans
|
|
|
What is aggrecan?
what is the large aggregate called? |
The predominant proteoglycan in cartilage.
Proteoglycan aggregates gives gel-like properties and resistance to deformation in cartilage. it binds at regular intervals to a central hyaluronan molecule |
|
|
What is one of the largest macromolecules known?
|
Proteoglycan aggreates formed cy aggrecan.
|
|
|
How does the plant cell wall have a similar role to the animal ECM?
|
The cell wall surrounding plnt cells serves many of the same functions as the extracellular matrix produced by animal cells, even though the two structures are cmoposed of entirely different macromolecules and have a different organization.
|
|
|
What are the functions of the cell wall?
|
1. withstand the osmotic turgor pressure of the cell
2. connects cells into tissues 3. signals a plant cell to grow and divide 4. controls the shape of the plant organs. |
|
|
What are the components of a cell wall?
|
1. Layer of cellulose micofibers
2. embedded in a matrix of pectin and hemicelluose 3. complete coats the outside of the plasma membrane 4. cellulose is the most abundant molcule in the cell wall. |
|
|
What are the structures of cell wall?
|
Microfibrils of cellulose are extensively crosslinked by hemicellulose polysaccharide chains.
Glucose linked together -> cellobiose -> glucan chain -> microfibril Layers of microfibrils preent the cell wall from strtching laterally. as a plant matures, it lays down an inner secondary wall |
|
|
What polysaccarides presnt in cell walls?
|
1. Hemicellulose
-cross-link cellulose microfibrils by hydrogen bonds - higly branched polysaccharides - help bind microfibrils to each otehr and other matrix components, particularly the pectins - assist in cel cushioning 2. Pectin - Contains multiple negatively charged sacharrides, that bind cations such cas Ca2+ and become highly hydrated (like hyaloronan) - when purified, it binds water and form a gel (ex. ice cream) - abundant in middle lamella |
|
|
what degrades pectin?
|
Pectinase found in detergent to remove grass stain.
|
|
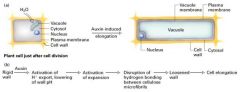
Acid-growh hypothesis
|
Auxin stimulate proton secretion at the "growing" end of the cell by activating a proton pump bound to the plasma membrane.
- the pH of the cell wall falls from 7 to 4.5 3. low pH activates a class of wall proteins called expansins that disrupt the hydrogen bonding b/w cellulose microfibrils, casuing the laminate structure of the cell wall to loosen. 4. with the rigidity of the wall reduced, the cell can elongate. |
|
|
What is auxin and its role in plants?
|
Auxin is a plant hormone which involves in bending toward light.
|
|
|
How are cellulose microfibrils synthesized and oriented?
|
cellulose synthase in the plasma membrane synthesizes new cellulose microfibrils on the exoplamis face.
- cortical microtubules underlying the membrane are thought to determine the orientation of the cellulose microfibrils. |
|
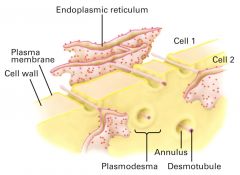
How is plasmodesmata differ from gap junctions in animals?
|
It's also used in cell communication.
- soluble molecules pass thru the cytosolic annulus, but membrane-bound molecules may pass from cell to cell via the desmotubule. - transport thru plasmodesmata is reversibly inhibited by an elevation in cytosolic Ca2+. |
|
Explain the cell cycle
|
Interphase -> longest
|
|
|
What drives the cell cycle?
|
1. Series of experiments in the 1970s suggested that the cell cycle is driven by specific chemical signals present in the cytoplasm.
2. mammalian cells grown in culture. 3. 2 cells in different phases of the cell cycle were fused to form a single cell with 2 nuclei 4. S + G1 -> S phase 5. M + G1 -> mitosis began |
|
|
What drives the cell cycle?
|
1. Series of experiments in the 1970s suggested that the cell cycle is driven by specific chemical signals present in the cytoplasm.
2. Mammalian cells grown in culture. 3. 2 cells in different phases of the cell cycle were fused to form a single cell with 2 nuclei 4. S + G1 -> S phase 5. M + G1 -> mitosis began |
|
|
Explain cell-cycle control system
|
1. Cyclically operating set of molecules in the cell that both triggers and coordinates key event s in the cell cycle (like an automatic washing machine)
2. Cell-cycle control proceeds on its own driven by a built-in clock. It also is subject to external adjustment and internal control 3. The cell is regulated at certain checkpoints by internal and external controls. |
|
|
Explain cell-cycle checkpoints
|
1. Critical points where stop and go-ahead signals can regulate the cell cycle
- G1 checkpoint (restriction point); most important; if the cell passes this point it will usually succeed in dividing - otherwise the cell will switch to a non-dividing state called G0 (Most cells of the human body are in this state) - G2 checkpoint - M checkpoint |
|
|
Explain cyclins and cyclin-dependent kinases
|
1. These are 2 main typoes of proteins involved in control of the cell cycle.
2. To be active, these kinases must be attached to a cyclin (protein that cyclically fluctuates in the cell) 3. The activity of a Cdk rises and falls with changes in the concentration of its cyclin partner. |
|
|
What is MPF?
|
1. The cyclin-Cdk complex that was discovered first; Maturation Promoting Factor (M-phase promoting factor)
2. Triggers the cell's passage past the G2 checkpoint into the M phase 3. MPF acts both directly and indirectly by causing the nuclear envelope to fragment by phosphorylating and stimulating other kinases to phosphorylate 4. In M phase, MPF turns itself off by initiating a process that leads to the destruction of its cyclin by degrading enzymes. |
|
|
Explain molecular basis of the cell-cycle control system at the G2 checkpoint
|
1. By the G2 checkpoint, enough cyclin is available to produce many molecules of MPF
2. MPF promotes mitosis by phosphorylating various proteins, including other enzymes 3. One effect of MPF is the initiation of events leading to the breakdown of its own cyclin 4. The Cdk component of MPF is recycled. Its kinase activity will be restored by association with new cyclin that accumulates during interphase. |
|
|
How is cyclins and Cdk are invovled in regulation of the cell cycle?
|
1. Altered regulation of expression of a least one cyclin as well as mutation of several proteins that negatively regulate passage through the restriction point can be oncogenic.
2. Overexpression of the proto-oncogene encoding cyclin D1 or loss of the tumor supressor genes encoding p16 and Rb can cause inappropriate, unregulated passage through the restriction point in late G1, a key element in cell cycle control. Such abnormalities are common in human tumors. |
|
|
What are some functions of TGFbeta proteins related to cancer
|
1. TGFbeta is secreted by many body cells and has a diverse reange of biological activities.
2.TGFbeta inhibits the growth of many types of cells, including most epithelial and immune system cells 3. Loss of TGFbeta-mediated growth inhibition -> development of tumors 4. One important gene induced by TGFbeta encodes p15 which ultimately ends in causing the cell to arrest in G1. |
|
|
What's cancer?
|
1. Mutations in TGFb receptors or Smad proteins
2. Many tumors contain inactivating mutations in either the TGFb receptors or the Smad proteins, making them resistant to growth inhibition by TGFb. 3. Human pancreatic cancers -> deletion in the gene encoding Smad4 4. Loss of type I or II TGFb receptors -> retinoblastoma, colon and gastric cancer, hepatoma, T- and B-cell malignancies. |
|
|
What is metasis?
|
It's a mutations in TGFb pathways affecting matrix proteins.
|
|
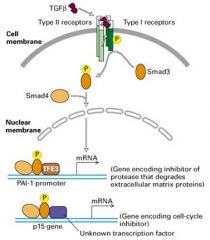
What is TGFb pathway?
|
1. This pathway induces expression of genes encoding many extracellular matrix proteins, such as collagens, plasminogen activator inhibitor (PAI-1) (inhibitor of a serum protease that degrades many matrix proteins)
2. Once the cell is unable to synthesize these proteins -> metastasis, allowing tumor cells to move freely since less matrix will be made by the surrounding cells and matrix that is present may be degraded the newly produces proteases. |
|
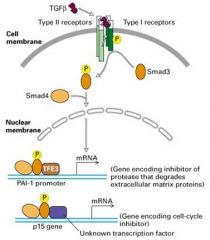
What is TGFb pathway?
|
1. This pathway induces expression of genes encoding many extracellular matrix proteins, such as collagens, plasminogen activator inhibitor (PAI-1) (inhibitor of a serum protease that degrades many matrix proteins)
2. Once the cell is unable to synthesize these proteins -> metastasis, allowing tumor cells to move freely since less matrix will be made by the surrounding cells and matrix that is present may be degraded the newly produces proteases. |
|
|
What is a tumor?
|
1. A mass of "unwanted cells" that is growing out of control.
2. Some tumors don't have serious consequences, but those composed of cells that spread throughout the body usually cause disease. |
|
|
What are some causes of cancer?
|
1. Mutations in somatic cells
2. Some are casued by inherited genetic mutations that predispose them to develop specific typoes of cancer 3. An individual cancer does not result from a single mutaion, but from an accumulation from 3-20 mutations, depending on the typoe of cancer, in genes that nromally regulate cell multiplication. |
|
|
What are metastatic tumor?
|
1. Benign tumors stay localized to the tissues from which they arose and pose very little health threat.
2. Malignant tumors usually invade surrounding tissues, get into the body's circulatory system and start to grow in other locations, posing a serious health threat |
|
|
What is metastasis?
|
Spread of tumor cells and establishment of areas of secondary growth.
|
|
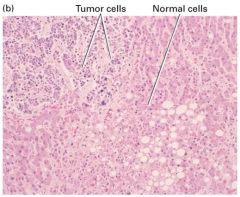
How are cancer cells and normal cells?
|
1. Less well differentiated
2. Characteristics of rapidly growing cells - High nucleus-cytoplasm ratio - Prominent nucleoli - Many mitosis - Relatively little specialized structure 3. Invasion of abnormal cells in an otherwise nromal tissue section. |
|
|
What are malignant tumor classification?
|
1. Carcinoma
- Tumors that derive from endoderm or ectoderm 2. Sarcomas - Tumors that derive from the mesoderm, include leukemia |
|
|
Alterations in cell-cell interactions are associated with malignancy
|
1. Basal lamina (underlies epithelial cells and surrounds endothelial cells of blood vessels) is a physical barrier that keep tissues separated
2. Metastatic cells break this (and other barriers) 3. Tumor cells often produce elevated levels of cell-surface receptors specific for the proteins and polysaccharides composing basal laminae (collagen, proteoglycans, glycoaminoglycans) and secrete enzymes that digest these proteins |
|
|
What is protease?
|
1. The protease is called plasminogen activator and it cleaves a peptide bond in the serum protein plasminogen, converting it to the active protease plasmin.
2. The increased levels of this protease promotes metastasis by helping tumor cells digest and penetrate the basal lamina |
|
|
Tumor growth requires formation of what?
|
Formation of new blood vessels. In order to grow a tumor needs a blood supply. Angiogenesis is the process whereby tumors induce the formation of new blood vessels that nourish it.
|
|
|
Explain angiogenesis
|
1. Degradation of the basal lamina that surrounds a nearby capillary.
2. Migration of endothelial cells lining the capillary into the tumor. 3. Division of these endothelial cells. 4. Formation of a new basement membrane (basal lamina) around the newly elongated capillary |
|
|
What are tumors that secrete growth factors that stiumulate angiogenesis?
|
1. Basic fibroblast growth factor (bFGF)
2. Transforming growth factor alpha (TGFa) 3. Vascular endothelial growth factor (VEGF) 4. Since new blood vessels are constantly forming during embryonic development, few normally form in adults so a specific inhibitor of angiogenesis (such as angiogenin, endostratin, or antagonists of the VEGF receptor) may be a good form of cancer therapy. |
|
|
What are some mystery/problems concerning angiogenesis andprimary tumor removal?
|
1. A primary tumor often secretes a substance that inhibits angiogenesis around secondary metastases
2. When the primary tumor is removed, the secondary tumors may be stiumulated to releas factors that stimulate angiogenesis; thus leading to the metastatic growth of these former secondary tumors. |
|

What is the purpose of transfection-cancer experiment?
|
To determine that mutations lead to differences b/w normal and cancer cells.
1. Transfection experiemtn with cultured mouse fibroblasts called 3T3 cells 2. These cells normally grow only when attached to the plastic surface of a culture dish and are maintained at low cell density -> cells in the G0 phase. 3. These cells can differentiate into a variety of cell types including fat and endothelial cells (lining blood vessels) 4. DNA from a tumor is added to 3T3 cells in culture 5. About 1 in a million cells incorporates a particular segment of the tumor DNA that causes a distinct phenotype 5. Progeny of these cells are more rounded and less adherent to each other and the dish, forming a 3D cluster of cells 7. These transformed cells have more properties in common with malignant tumors 8. After the transforming the cells, the specific DNA segment that causes the transformation was cloned. 9. The cloned segment contained a Ras gene designated RasD; Remember Ras participates in many intracellular transduction pathways activated by growth factors; it is inactive with bound GDP and active with bound GTP. |
|
|
What are the properties of transformed 3T3 cells?
|
1. Changes in cell morphology
2. Ability to grow unattached to a basal lamina or other ECM 3. Reduced requirement for growth factors 4. Secretion of plasminogen activator 5. Loss of actin microfilaments |
|
|
What effect does RasD have on intracellular pathways?
|
It hydrolyzes bound GTP very slowly; so GTP accumulates in the active sites, sending a grwoth-promoting sequence to the nucleus; even in the absence of hormones that are normally required.
|
|
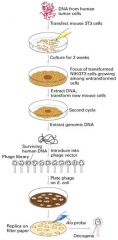
What happen in transformed 3T3 cells?
|
1. Loss-of-function mutation in the p16 gene
2. P16 gene encodes a cyclin-kinase inhibitor that restricts progression through the cell cycle |
|
|
How can you identify and molecular clone the RasD oncogene?
|
1. Addition of DNA from a human bladder carcinoma to a culture of mouse 3T3 cells
2. 1/1,000,000 cells transforms and forms a "focus" 3. The oncogene is cloned by isolating DNA from the initial focus and separating from other DNA by a secondary transfer into mouse cells. 4. This DNA from the secondary transfer is cloned into a phage which hybridizes with an Alu probe (nearby repetitive DNA sequence) 5. The phage contains all or part of the transforming oncogene. |
|
|
What is the difference between an oncogene and a proto-oncogene?
|
An oncogene is any gene such as RasD that encodes a protein capable of transforming cells in culture or inducing cancer in animals
2. The normal cellular gene from which it arises is called a proto-oncogene |
|
|
What is onco gene?
|
cancer gene
|
|
|
Normal form of cancer gene is called?
|
Proto oncogene
|
|
|
What chemicals move S+G1 -> S phase, M+G1->mitosis
forward? |
Cyclins and CDK (cyclin dependent kinase)
|
|
|
What are cell-cycle checkpoints? Give examples
|
Checkpoint is a critical point where stop and go-ahead signals can regulate the cell cycle.
1. G1 Checkpoint (restriction point) is MOST important; if the cell passes this point it will usually succeed in dividing - otherwise the cell will switch to a non-dividing state called G0 2. G2 checkpoint 3. M checkpoint |
|
|
What are 2 molecules relates to cell-cycle checkpoints?
|
Cyclins and cyclin-dependent kinases
|
|
|
What is Maturation Promoting Facor?
|
Famous cyclin-Cdk complex.
Triggers after G2 and right before M phase begins. |
|
|
How is MPF turned off?
|
By itself
|
|
|
If there are problems with cyclins and cdk, what happens to cell cycle?
|
It alter the regulation of the cell cycle
|
|
|
Problem in cyclin+Cdk -> missing check points -> uncontrolled cell cycle -> cause of cancer.
|
.
|
|
|
What are some molecular examples of changes in cells that cause cancer?
|
Hallmark of cancer
|
|
|
What's the difference between benign tumors and malignant tumors?
|
1. Benign tumors stay localized to the tissues from which they arose and pose very little heath threat.
2. Malignant tumors usually invade surrounding tissues, get into the body's circulatory system and start to grow in other locations, posing a serious health threat. |
|
|
Once the primary tumor is removed, why do more secondary tumors grow?
|
Because there is another signal that is sent out to inhibit blood vessel formation.
|
|
|
What are the functions of Endostatin and Angiostatin?
|
Inhibit the Angiogenesis
|
|
|
What's the difference between oncogene and proto-oncogene?
|
An oncogene is any gene such as RasD that encodes a protein capable of transforming cells in culture or inducing cancer in animals
The normal cellular gene from which it arises is called a proto-oncogene |
|
|
What is APC gene?
|
Adenomatous polyposis coil gene, basically form of polyp in your colon.
|
|
|
What are the 4 types of mutations seen in additional mutation of malignant cell?
|
1. Active K-Ras protein
2. Loss of tumor supressor gene, DCC 3. Loss of tumor supressor gene, p53 4. APC gene (bump in your colon) |
|
|
What are 4 types of cancer vaccines?
|
Cytokine vaccine, CEA vaccine, HSP vaccines, Telomerase vaccine
|

