![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
369 Cards in this Set
- Front
- Back
|
What is Trypsin?
|
A protease enzyme that chews apart cell membrane proteins and makes cells less cohesive.
|
|
|
What is EDTA?
|
A divalent cation chelator, it depletes free CA2+ an important part of cell adhesion.
|
|
|
What is a primary cell culture?
|
Cells taken directly from an organism, display contact inhibition and divide a limited number of times.
|
|
|
What's the 'Hayflick Limit.'
|
The maximum number of times a cell line can replicated before undergoing senescence, usually about 50.
|
|
|
What is a cell line?
|
Cells which are transformed and are able to grow indefinitely, often don't display contact inhibition or properly regulated growth.
|
|
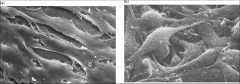
Which one of these is the transformed Fibroblast?
|
The one on the right.
|
|
|
What is a blastocyst?
|
A structure formed in the early gestation of vertebrates, it contains an inner cell mass that eventually becomes the embryo.
|
|
|
Embryoid bodies contain features of what three germ lines?
|
Endoderm (gut lining), mesoderm (cartilage) and ectoderm (neural precursors).
|
|
|
What two types of cells make up the 'stem cell niche' in the crypts of intestinal epithelial cells?
|
Mesenchymal cells and paneth cells.
|
|
|
What four genes need to be introduced to fibroblasts to reprogram them to 'induced pluripotent cells?'
|
Oct4, Sox2, Klf4 > (genes characteristic of ES cells) and c-Myc >(frequently expressed in cancer cells).
|
|
|
What is the typical magnification of a Bright-field Microscope?
|
40-1000x
|
|
|
What is resolution?
|
The ability to distinguish between two very closely positioned objects as separate entities.
|
|
|
What's the smallest distance a regular microscope can resolve?
|
0.2 microMetres
|
|
|
Which is better a greater or smaller resolution?
|
Smaller.
|
|
|
D = 0.61 λ / N sin α
Define all of the components of this equation. |
D = distance resolved between two points.
λ = wavelength of light N = refractive index of medium between the specimen & objective lense. Nsinα = angle of aperture α = 1/2 angle of light entering object. |
|
|
Based on the resolution equation what can you do to get a better resolution?
|
Increase the angle of light, decrease the wavelength, increase the refractive index of the medium.
|
|
|
What is the spectrum of visible light?
|
400 - 700 nm
|
|
|
What is phase contrast microscopy used for?
|
To examine live 'unstained' cells, it uses two wavelengths out of line?
|
|
|
What is differential interference microscopy used for?
|
Live 'unstained' cells, uses polarized light to show thickness & refractive index, defines outlines of large organelles and cell edge well.
|
|

What types of microscopy are these? (From left to right)
|
Bright Field, phase contrast, DIC.
|
|
|
Define Aneuploid
|
A cell with an abnormal number of chromosomes.
|
|
|
What is a condenser microscope?
|
A light microscope using two or more lenses (increasing the magnification properties).
|
|
|
What does the condenser do?
|
It helps to focus the light from the light source on to a specimen.
|
|
|
What does the objective lens do?
|
It collects and focuses light that passes through the specimen, achieves a magnification of 100X.
|
|
|
How much does the ocular lens magnify the specimen?
|
10x
|
|
|
What do polarizers do?
|
Allow specific planes of light through, (horizontal or vertical).
|
|
|
What is a fluorophore?
|
It is a chemical that when its outer electrons are excited by a certain wavelength of light, they consecutively return to their original ground state, emitting a certain wavelength of light in doing so.
|
|
|
What is the 'Stokes shift?'
|
The difference between the optimal and excitation wavelengths.
|
|
|
What does an emission filter do?
|
Refines the emitted light to a defined wavelength.
|
|
|
What do DAPI and Mitrotracker Red do?
|
Stain the nucleus blue, and mitochondria red.
|
|
|
What is Immunofluorescent staining?
|
Imagining dye can be conjugated with antibodies to localize any molecules of interest (particularly proteins) in cells.
|
|
|
What is the secondary antibody in immunofluorescent imaging?
|
The antibody that binds the first antibody released by the animal, the one that recognizes a certain protein.
|
|
|
What is phalloidin?
|
A toxin that binds readily to the protein actin.
|
|
|
What is a chromophore? What is a common example of a chromophore?
|
A sequence of amino acids that under certain light conditions will fluoresce, eg. GFP
|
|
|
Principles Of Confocal Microscopy:
|
Confocal microscopy uses a laser (which uses a very precise wavelength of light) that enters a pinhole, which focuses that emitted light on a particular point in the cell. The emitted light (from the cell) is focused through a complementary pinhole that ultimately will be detected. In this case, only a focal plane (Z-layer) is being illuminated.
|
|
|
What is a Z stack?
|
Images taken at different focal planes.
|
|
|
What is deconvolution?
|
A computational restoration method (using the point spread function algorithm) that provides an alternative means of producing high-resolution images.
|
|
|
What is Fluorescence Resonance Energy Transfer (FRET)?
|
A method to measure protein interactions in live cells. If no protein interaction occurs then the excitation of cyan fluorescent protein (CFP) will only result in cyan fluorescence (480 nm). However, if protein interaction occurs then the excitation of CFP will result in yellow fluorescence (535 nm) of another protein.
|
|
|
How does electron microscopy work?
|
The specimen is stained with electron dense heavy metals, a heated wire filament is used to provide electrons, magnetic condensers focus these electrons on the specimen and electrons are caught by the heavy metal on the specimen, preventing transmission. This can only be done on dead cells, and gives a resolution as small as 1 nm.
|
|
|
What are the two types of electron microscopy?
|
Scanning electron microscopes (SEM) and transmission electron microscopes (TEM), in TEM the cross section of a cell is obtained by detecting the eletrons that pass through a cell, SEM gives images that appear to be 3D by detecting electrons emitted from heavy metal coated specimens.
|
|
|
What is the equation for resolution in electron microscopes?
|
D = 0.61 λ / α
|
|
|
How does Fluorescent Activated Cell Sorting (FACS) work?
|
Live cells are labeled with fluorescent antibodies these cells pass single file through a laser light beam, the fluorescent light is detected and the cells are passed through a nozzle, given a charge based on the degree of fluoresence detected, they are then separated based on this charge and collected.
|
|
|
What is differential centrifugation?
|
Centrifuging a homogenate at different speeds and times, measures cell organelles which differ in size and mass.
|
|
|
What is Equilibrium Density-Gradient Centrifugation?
|
A type of centrifugation that separates the homogenate based on density when applied to a gradient of sucrose solution. Over time organelles will migrate to the sucrose layer equal to their own density and remain there.
|
|
|
What are non-ionic detergents (eg. Triton X-100) used for?
|
Disrupting the cell membrane (lipid bilayer) and preserving the function of any proteins.
|
|
|
What are ionic detergents, (eg. SDS) used for?
|
Disrupting the cell membrane as well as for the denaturing (disruption of hydrogen and ionic bonds) of proteins.
|
|
|
What is SDS PAGE (polyacrylamide gel electrophoresis) used for?
|
The electrophoretic separation of proteins (seeing & quantifying proteins. )
|
|
|
What is western blotting used for?
|
Detecting specific proteins, SDS page separates proteins, the first antibody binds to the protein, the second antibody w/ an enzyme binds to AB1 and then the enhanced chemiluminescence substrate reacts with the enzyme to create a detectable glow.
The intensity of the band created reflects the amount of protein present. |
|
|
What is 'targeting,' what gets targeted?
|
Directing proteins to the right organelles during or after synthesis, uses signal sequences, Usually done by proteins created in the cytosol by cytosolic ribosomes. They are usually targetted to mitochondria, chloroplasts, peroxisomes, and the nucleus.
|
|
|
What is 'sorting' and what gets sorted?
|
Proteins that need to be moved down a SECRETORY pathways (to the ER, Golgi, and/or lysosomes) these are usually synthesized by ER ribosomes.
|
|
|
What do translocation channels do?
|
help secretory proteins cross the pesky hydrophobic membrane
|
|
|
What is a microsome?
|
Open membrane of RER that reforms after homogenization.
|
|
|
What happens to proteins in the RER?
|
Secreted and membrane bound proteins are sorted, post translational modifications such as adding of sugars, disulphide bonds are created, chaperones assist the polypeptides in folding.
|
|
|
Are proteins imported into the RER or are they made into it?
|
Translocation of secretory proteins into microsomes is coupled to translation.
|
|
|
What are glycoproteins?
|
Proteins with attached carbohydrates
|
|
|
What is glycosylation and what is the purpose?
|
The enzymatic transfer of 14-residue oligosaccharide precursor from dolichol carrier (a glycolipid) to an ASPARAGINE (Asn) residue of a nascent polypeptide by oligosaccharyl transferase.
Most secretory proteins are glycosylated to make them “sticky” or charged that makes cells adhere to each other better. Glycosylation also confers proper folding of proteins in the RER and rids cell of improperly-folded proteins. |
|
|
What does signal peptidase do?
|
It cleaves the signal sequence (proteolytic cleavage?)
|
|
|
What does Protein disulphide isomerase (PDI) do?
|
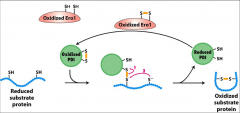
It catalyzes disulphide bond formation between the SH groups of two cysteine residues in newly forming polypeptides.
|
|
|
What is Ero1?
|
A protein necessary for PDI regeneration. PDI is converted to it's reduced form when catalyzes disulphide bond formation, Ero1 converts it back to its oxidized state.
|
|
|
What is Bip?
|
A chaperone that transiently binds to a nascent polypeptide chain in the RER to assist in proper folding.
|
|
|
What are calnexin and calreticulin?
|
Two lectins that bind transiently to certain oligosaccharide chains promoting proper folding of adjacent segments. They work together with Bip in the RER.
|
|
|
What is Hsc70?
|
A chaperone that keeps cytosolic proteins in an unfolded or partially folded state so it can still be imported into the mitochondrial matrix. It's a 'matrix chaperone' it can hydrolyze ATP to help push the protein into the mitochondria after it binds to an import receptor and is moved into the general transport pore...
|
|
|
What is the mitochondrial matrix targeting sequence?
|
20- 50 amino acids at N-terminus rich in both hydrophobic, basic and hydroxylated amino acids (amphipathic). It is recognized by Tom20/22 to help move the nascent polypeptide move into the Tom 40 general import pore.
|
|
|
What is receptor mediated endocytosis?
|
A method of selective internalization of specific extracellular molecules (ligands.)
Useful for LDL (low-density lipoprotein) transferrin, hormones (e.g. insulin), certain glycoproteins, |
|
|
What does the golgi complex do?
|
It processes and sorts secretory, membrane and lysosomal proteins, itconsists of flattened, disk-like cisternae (stacked tubules) with no ribosomes.
|
|
|
What are the three types of cisternae in the golgi?
|
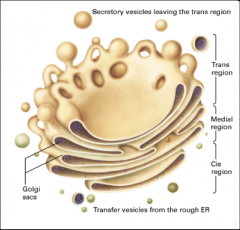
Cis-Golgi (CGN), medial-Golgi and trans-Golgi (TGN). This gives us two flanked networks of tubules: the CGN faces the RER and the TGN is opposite the RER (faces the plasma membrane).
|
|
|
How are things transported to and from the golgi/ER?
|
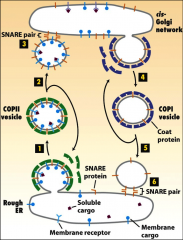
With vesicles.
|
|
|
What do coat proteins do?
|
Promote the budding of vesicles, they bind to the cytosolic domain of membrane cargo proteins,
|
|
|
What do GTP binding proteins do in the ER?
|
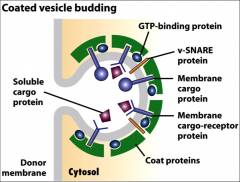
The small ones initiate budding of vesicles when they are recruited to a patch of donor membrane, they control assembly and disassembly of COPII coat proteins.
They also help the naked vesicle dock to target membrane. |
|
|
What do membrane cargo proteins do in the ER?
|
They work as receptors that bind soluble proteins in the lumen, and their cytosolic domain binds coat proteins.
|
|
|
What do snare proteins do?
|
Promotes fusion of vesicles with target membranes. A vesicle is covered in V-snare proteins that are exposed when the coat proteins are shed from the vesicle. These Vesical Snare proteins interact with the Target Snare proteins on cis-Golgi by pairing, the vesicle fuses, releasing its contents into the golgi complex.
|
|
|
What do COPI coat proteins do?
|
Participate in retrograde transport from the golgi back to the RER.
It is used to recycle the membrane bilayer and certain proteins, such as v-SNAREs and wrongly-sorted ER-resident proteins. |
|
|
What is DXE?
|
A cargo membrane protein recognized by COPII proteins.
|
|
|
What is required for dissociation of SNARE complexes?
|
ATP hydrolysis.
|
|
|
How is cystic fibrosis related to vesicle creation?
|
Mutations in deltaF508 affect the ability of CFTR to bind COPII coat proteins, because of this CFTR remains in the ER and is degraded. ** CFTR is still functional, but incorrectly sorted.
|
|
|
What is the KDEL Sorting Signal?
|
A sorting signal found on ER luminal proteins that signals they should be transported BACK to the ER if they happen to end up in the golgi complex.
It's is present in COPI and COPII |
|
|
How is KDEL controlled?
|
The KDEL sorting signal is very susceptible to changes in pH, the lower pH in the golgi favors binding and the higher pH in the ER promotes release of KDEL bearing proteins.
|
|
|
What is clathrin?
|
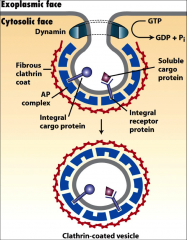
A coat protein that coats vesicles coming from Trans-Golgi Network or the PM (depending on 1 or 2) that fuse with late endosomes to be transported to lysosomes.
|
|
|
What are AP complexes?
|
Additional proteins often found on clathrin vesicles, some vesicles coated in AP complexes fuse directly with the lysosome. They recognize the the sorting signals of cargo proteins or receptors. They can move things to the PM or the TGN.
|
|
|
What are M6P residues?
|
Sorting signals (carbohydrate residues) that direct proteins to Iysosomes, it is generated in the cis-Golgi, an example of an enzyme/protein that would be tagged by this is acidic hydrolase.
In trans golgi, any proteins bearing M6P interact with M6P receptors in the membrane and are directed to clathrin/AP1 vesicles. ** late endosomes have a low pH that cause M6P receptors to detach & be recycled. |
|
|
Rank these from highest pH to lowest pH; the ER, the golgi complex, late endosomes.
|
They're in order.
|
|
|
What are lipoproteins?
|
Large well-defined water-soluble complexes/particles that transport lipids.
|
|
|
What are LDLs?
|
Low density lipoproteins they mediate cholesterol transport, they have a polar surface and an apolar core.
-Amphipathic shell, composed of a phospholipid monolayer, and apolipoprotein. -Apolar core – hydrophobic, mostly cholesteryl esters |
|
|
What are lysosomal storage diseases? Name 1 example.
|
The result of the absence of one or more lysosomal enzyme causing the accumulation of undegraded material in the lysosome. inclusion-cell (I-cell) disease is an example.
|
|
|
What are the three types of endocytosis?
|
Phagocytosis - large particles are internalized using actin remodelling.
Pinocytosis - non specific small molecules/droplets taken up. Receptor mediated endocytosis, very specific binding of large ligand by the PM, which buds inwards and pinches off to make a transport vesicle. |
|
|
What is ApoB?
|
A single molecule that wraps around LDL molecules apolioprotein B. Helps receptors in the PM recognize it and internalize it with clathrin coated vesicles.
|
|
|
Where does AP1 direct vesicles?
|
It's an adapter protein found on PM vesicles that direct it to the trans golgi network.
|
|
|
Where does AP2 direct vesicles?
|
It's an adapter protein found on TGN vesicles that direct it to the plasma membrane.
|
|
|
What does dynamin do?
|
Pinches off clathrin coated vesicles with GTP hydrolysis.
|
|
|
What are the three domains of the LDL receptor?
|
Short C-terminal cytosolic segment with sorting signal
The Long N-terminal exoplasmic segment with a ligand binding domain The b-propeller domain |
|
|
What happens to the LDL receptor at low pH?
|
The high affinity of the ligand binding arm with apoB is lost and the histidine residues in the beta propeller domain become protonated, binding to the ligand binding arm, freeing LDL. This occurs in the endosome.
|
|
|
How are late endosomes and lysosomes acidified?
|
v-class proton pumps work to transport H+ across membranes via an ATP- dependent mechanism. Chloride channels are also present on the lysosomal and late endosomal membranes. These anions passively follow the pumped protons resulting in acidification (creation of HCl) of the lumen.
|
|
|
What does Transferrin do?
|
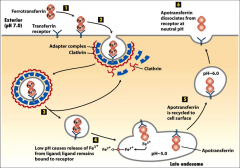
It transfers iron to all tissue cells, it's a dimer that can bind two Fe3+ molecules at once.
|
|
|
What is ferrotranferrin?
|
The form of transferrin that is bound to iron.
|
|
|
What kind of vesicles is ferotransferrin transported in?
|
'Clathrin coated pits;' when it is bound to the transferrin receptor, AP2 is present on the vesicle because it comes from the PM and goes to the lysosome. It comes from the PM and when it connects to the late endosome the pH drop causes iron to be released.
|
|
|
What is apotransferrin?
|
Transferrin lacking iron.
|
|
|
What is autophagy?
|
Self eating, performed by hydrolases in the lysosome. It mediates the turnover of long-lived proteins and excess or abnormal organelles. Considered a 'lysosomal/vacuolar degradative pathway that is conserved in eukaryotic organisms.'
It involves a double membrane vesicle formed in the cytosol. |
|
|
What are the proteins ATG 8, 12, 5, 16, essential for?
|
Extending the membrane created in autophagy. A naked autophagosome has these cleaved off.
|
|
|
What effects does loss & overexpression of ATG 8 have in fruitflies?
|
Loss of ATG8 results in progressive neurodegeneration and early death, overexpression of ATG8 results in extended lifespan.
|
|
|
What effect does loss of ATG 5 have on mice?
|
Loss of Atg5 expression in the brains of mice leads to progressive neurodegeneration.
|
|
|
What are the four stages of the cell cycle?
|
G1 after mitosis, before synthesis, the longest phase.
S the DNA is replicated X2, G2 there is double the DNA as G1 Mitosis - the cell splits. |
|
|
What are the stages of mitosis, what happens in each?
|
Prophase -the nuclear envelope breaks down, microtubules form the mitotic spindle apparatus, and chromosomes condense.
Metaphase -attachment of chromosomes to microtubules via their kinetochores is complete (align at metaphase plate). Anaphase -microtubule motors and shortening of spindle microtubules pull the sister chromatids toward opposite spindle poles. Telophase/cytokinesis -chromosomes decondense, and cells reassemble nuclear membranes around the daughter cell nuclei and undergo cytokinesis (cell division). |
|
|
What is the difference between budding and fission yeast?
|
Budding yeast grow a bud that pinches off into a new yeast cell, fission yeast grow longer and form a wall "septum."
|
|
|
What does cell cycle control involve?
|
Three important protein families (2 of which are enzymes): kinases (that phosphorylate), phosphatases (that dephosphorylate) and cyclins (that interact with kinases to regulate them).
|
|
|
What does the cdc 28 gene encode?
|
Cyclin Dependent Kinase (CDK), the ONLY CDK in S Cervisae.
|
|
|
What does CDC 2 encode, and what effects does it's mutation have?
|
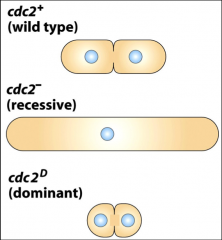
The CDK of S. POmbe yeast. Loss of Cdc2 activity (recessive) prevents S.pombe from entering M phase
Gain of Cdc2 activity (dominant) brings on mitosis earlier |
|
|
What is cdc 13?
|
The gene that encodes mitotic CYCLIN, it forms a dimer with cdc 2 (Kinase) to make the MITOSIS PROMOTING FACTOR (MPF)
|
|
|
What do cyclins do?
|
Control the activity of kinases involved in the cell cycle. They are controlled by their rate of transcription and their rate of degradation by ubiquitin-mediated proteasome.
|
|
|
What is ubiquitin?
|
A 76 AA protein found in all prokaryotic cells, its used to tag proteins for degradation by proteosomes. 'ubiquitination.' It is added by ubiquitin ligases
|
|
|
What is Wee1? What does it affect?
|
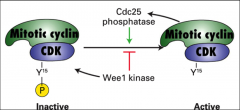
A kinase that phosphorylates CDK leading to its inactivation. It regulates MPF.
Specifically it introduces a phosphate group to the tyrosine at position 15 (Y15) on bound CDK. |
|
|
What is cdc 25?
|
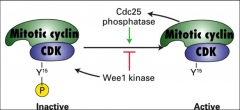
A phosphatase that removes the inhibitory phosphate group from CDK and promotes its ACTIVATION, it regulates MPF.
|
|
|
What occurs with an excess of cdc 25 or a deficiency of wee 1?
|
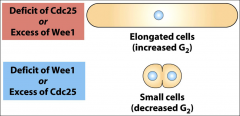
Elongated cells.
|
|
|
What makes up a ubiquitin ligase?
|
Two domains, one for binding the target protein and another for binding the ubiquitin conjugating enzyme, which attaches multiple ubiquitin molecules onto the target protein (polyubiquitination).
|
|
|
What does CAK do?
|
It's a cyclin activating kinase that phosphorylates the threonine at position 161 (T161) on bound CDK, so the MPF complex is somewhat activated. (The phosphate group at Y15 must still be removed.)
|
|
|
What needs to happen for MPF to be active?
|
The cyclin from gene cdc 13 and the kinase from gene cdc 2 need to form a dimer, (the MPF itself) this is phosphorylated at Y15 immediately by Wee1 so cdc 25 needs to remove this phosphate group, as well the T161 must be phosphorylated by CAK. This makes it fully active to promote mitosis.
|
|
|
What needs to happen for MPF to be inactivated?
|
At ANAPHASE the cyclin in MPF is polyubiquinated and rapidly degraded. The ubiquitin ligase involved in this is the Anaphase Promoting COmplex/cyclosome (APC/C).
|
|
|
What is (APC/C)?
|
A ubiquitin ligase it polyubiquinates cyclin in MPF and rapidly degrades it. It recognizes a 9 AA 'destruction box.'
|
|
|
What do proteasomes do?
|
Degrade proteins marked by ubiquitin.
|
|
|
What does cdh1 do?
|
Ensures the activity of APC/C. (the ubiquination of MPF cyclin and degradation).
|
|
|
What does SCF do?
|
Degrades G & S cyclins.
|
|
|
What is p53 and what does it play a role in?
|
A transcription factor that guards the genome. DNA damage causes it to be PHOSPHORYLATED and STABILIZED, when it's activated it activates in turn the transcription of P21.
** it's activated by the kinase ATM it increases the expression of PUMA leading to apoptosis. |
|
|
What is p21?
|
A gene that codes for CDK inhibitor protein (CIP). It inhibits all cyclin-CDK complexes. and BRINGS THE CELL CYCLE TO A HALT. It is activated by phosphorylation of p53 (from DNA damage). When it is transcribed the DNA is either repaired or the cell undergoes apoptosis.
|
|
|
What are CDKi's?
|
Cyclin-dependent kinase inhibitors. They inhibit Cdk activity by physically blocking activation or substrate/ATP access (i.e. p21/CIP).
|
|
|
What are the basic components of biomembranes?
What properties are specific to biomembranes/ |
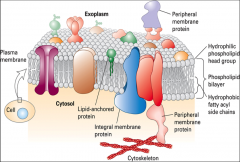
Lipids, Sterols, and Proteins. They are fluid, have closed compartments, are semi-permeable, and are asymmetric
|
|
|
What effects the melting temperature of fatty acids?
|
The length of the chain increases the melting temperature, the units of unsaturation (C=C) decreases the melting point.
|
|
|
What is a fatty acid?
|
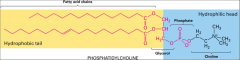
A long hydrocarbon chain attached to a polar carboxyl head group it's amphipathic.
They establish a balance between fluidity and solidity of the cell membrane with unsaturation. |
|
|
What are the three classes of membrane lipids?
|
Phosphoglycerides, sphingolipids, and sterols.
|
|
|
What are phosphoglycerides?
|
It is a membrane lipid based on the glycerol molecule, fatty acids are added to 2 spots on glycerol by ester linkages, the last spot on glycerol is bound to a variable polar head group.
|
|
|
What are sphingolipids?
|
A membrane lipid based off sphingosine, its an amino alcohol with a long hydrocarbon chain and a fatty acyl chain bound to the hydrocarbon chain with an amide bond.
|
|
|
What is a plasmologen?
|
A less common type of phosphoglyceride that has one of its fatty acids not linked to glycerol by an ester group.
|
|
|
What is a glycolipid?
|
A sphingolipid with a sugar for it's head group.
|
|
|
What does the head group of a lipid do?
|
It determines what the membrane will bind.
|
|
|
What is a phospholipid?
|
A head group that is attached to a phosphate, it's a very general term that can be a phsophoglyceride or a sphingolipid.
|
|
|
What are sterols?
|
Four hydrocarbons rings, cholesterol is the main sterol found in animals.
|
|
|
What effect does cholesterol have on the plasma membrane?
|
It increases the thickness, making it more difficult for things to passively diffuse across it. It can also affect the curvature of the membrane. (however sometimes it makes sphingolipids more fluid, it's effect is membrane dependent.)
|
|
|
What is a 'leaflet?'
|
One half of the bilayer, there is an inner and outer leaflet.
|
|
|
What are the two types of two dimensional movement in the plasma membrane?
|
Rapid lateral diffusion & slow transverse.
|
|
|
What is rapid lateral diffusion?
|
The movement along one leaflet, phospholipids are quickly exchanging positions in an adjacent fashion, this is the faster form of movement because it doesn't require flip-flop between leaflets.
|
|
|
What is slow transverse movement?
|
Flip-flop of phospholipids between leaflets, it's rare that it occurs on its own in cells, it usually must be actively carried out by the cell.
|
|
|
How can the fluidity of a membrane be measured?
|
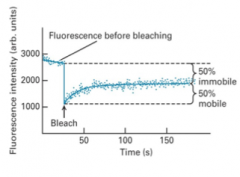
By fluorescently labeling proteins and observing how they move. Fluorescence Recovery After Photobleaching (FRAP.) The percentage recovery seen is the % mobile.
Membrane bound proteins are fluorescently labeled and photobleached... |
|
|
What are the internal & external faces of the plasma membrane?
|
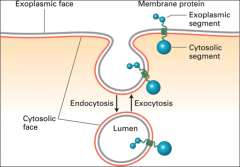
The internal face is the cytosolic face, the external is the exoplasmic.
|
|
|
What organelles have a double membrane bilayer?
|
The nucleus, mitochondrion, and chloroplast. Each bilayer is made of two leaflets.
|
|
|
What happens in exocytosis?
|
An intracellular vesicle fuses with the plasma membrane, and the lumen of the vesicle (exoplasmic face) connects with the extracellular medium.
|
|
|
What are some examples of asymmetry in biomembranes?
|
Phospholipid composition differs between leaflets, carbohydrates are found exclusively on the exoplasmic face, proteins are either embedded in the bilayer in a fixed orientation or are associated with only one side, etc.
|
|
|
What are the three types of membrane proteins?
|
Integral - asymmetric, specifically oriented protein molecules that are permanently embedded in the plasma membrane.
Lipid linked -proteins anchored to the plasma membrane by a lipophilic adduct. These proteins do not enter the bilayer (they are either present in the cytosol or in the extracellular medium), but are capable of lateral mobility in the membrane. Peripheral -attached to the plasma membrane non-covalently (not directly) through a variety of ways (e.g. ionic bonds, hydrogen bonds, protein-protein interactions, van der Waal’s forces, etc.). |
|
|
What are the main components of lipid linked membrane proteins?
|
A cytoplasmic (hydrophilic) domain, transmembrane (hydrophobic) domain and an exoplasmic (hydrophilic) domain.
|
|
|
What the two common types of transmembrane domains in lipid linked membrane proteins.
|
α-helices and β-barrels. α-helices consist of a row of about 20-25 hydrophobic amino acids that span the lipid bilayer.
|
|
|
What do positively charge amino acids do in integral membrane proteins?
|
They help anchor the protein by interacting with polar head groups in the membrane. Arg & Lys
|
|
|
What are GPI anchors, what are their components and what uses them?
|
Glycosylphosphatidylinositol anchors, they are proteins that link lipids, they are always exoplasmic, they consist of two components: phosphatidylinositol (phosphoglyceride present in the PM) and sugar residues (link phosphatidylinositol to membrane protein).
N-CAM proteins of the Ig superfamily utilize GPI anchors. |
|
|
How can association be achieved in lipid linked proteins?
|
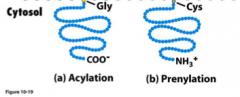
Acylation and Prenylation.
Acylation attaches a glycine residue at the N-terminus of the protein to an existing lipid in the membrane, while Prenylation attaches a cysteine residue at or near the C terminus of the protein. |
|
|
What are peripheral membrane proteins?
What's an example of one? |
are attached to the plasma membrane non-covalently (not directly) through a variety of ways (e.g. ionic bonds, hydrogen bonds, protein-protein interactions, van der Waal’s forces, etc.). Peripheral membrane proteins occur both in the cytosol (often associated with cytoskeletal filaments) and in the extracellular medium (often associated with ECM compoments). Dystrophin is a prime example of a peripheral membrane protein.
|
|
|
What are topogenic sequences?
|
Segments of a protein that ensure it acquires the proper orientation during its insertion into the endoplasmic reticulum. They direct the insertion of some single-pass transmembrane proteins, they are recognized generally by shape & charge, not the actual residue sequences.
|
|
|
What is an N terminal sequence?
|
A topogenic (cleaved) signal sequence found on type 1 integral membrane proteins.
|
|
|
What are type 1 membrane proteins?
|
Intergral proteins with an N term sequence and an STA sequence, their N terminal is found in the exoplasm/lumen, the c terminal is found in the cytoplasm. A good example is cadherins.
|
|
|
What are the four types of topogenic sequences available for transmembrane proteins?
|
The N-terminal (cleaved) signal sequence, the Stop-transfer/membrane anchor sequence (STA), the Signal-anchor-Internal (uncleaved) sequence (SA), and the Hydrophobic C-terminus.
|
|
|
What are tail anchored proteins? What is an example of one?
|
A membrane protein that only has a transmembrane domain (the C-terminus) and a cytosolic domain (the N-terminus). It contains the hydrophobic c terminus sequence. A good example of a tail anchored protein is a snare protein.
|
|
|
What does GET3 do?
|
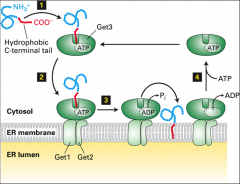
It's a protein that recognizes the C terminal of a tail anchored protein. With the help of GET1 GET2 and ATP hydrolysis it inserts the C term of the tail anchored protein into the ER.
|
|
|
What are SRP's?
|
Signal Recognition Particles that recognize N term signal sequences of Type 1 proteins, it also directs the ribosome/nascent chain complex to the ER membrane.
|
|
|
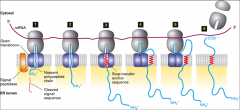
How are type 1 proteins embedded into the ER membrane?
|
First they are synthesized in the cytosol, the N term sequence is recognized by SRP's, the SRP's transport it through the translocon where the signal is cleaved? After the N-terminal signal sequence is cleaved in the ER lumen, the chain is elongated until the hydrophobic stop-transfer anchor sequence is synthesized and enters the translocon, where it prevents the nascent chain from extruding farther into the ER lumen. The stop- transfer anchor sequence then moves laterally between the translocon subunits and becomes anchored in the phospholipid bilayer. As synthesis continues, the elongating C-terminus chain may loop out into the cytosol.
|
|
|
What are type II proteins?
|
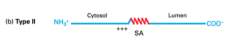
Integral membrane proteins with their N terminal in the cytosol and their C terminal in the exoplasm/lumen. Their orientation comes from positively charged residues on the N-terminus side of the SA sequence.
|
|
|
What are Type III proteins
|
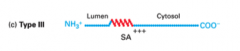
Integral membrane proteins with their C terminal in the cytosol and their N terminal in the exoplasm/lumen. Their orientation comes from positively charged residues on the C-terminus side of the SA sequence.
|
|
|
What are type IV proteins?
|
Integral membrane proteins that have MULTIPLE transmembrane domains. They have alternating SA and STA sequences, their initial orientation is controlled by the location of positive amino acids relative to their first topogenic sequence (this is ALWAYS a signal anchor sequence).
|
|
|
What does a signal anchor do?
|
It signals to transfer into the ER. If there are two present at the beginning of a type IV protein, that protein begins in the lumen, if there is only 1 it begins in the cytosol, Type II, III, and IV always have & start with a signal anchor.
|
|
|
Where are the charge amino acids always found?
|
On the cytosolic side.
|
|
|
What does a stop transfer anchor do?
|
Tells the mRNA to stop transferring into the ER, it's present in Type I proteins and Type IV proteins,
|
|
|
What is needed for something to passively diffuse across the membrane?
|
It needs to be small and uncharged, molecules also need to be relatively aqueous, but also slightly-soluble in lipids in order to cross the plasma membrane.
The concentration gradient also effects movement. |
|
|
What is K? What equation represents it?
|
The partition coefficient, it's a measure of lipid solubility, it is represented by K = c^m / c^aq. The higher K is a molecule has, the faster it will diffuse.
|
|
|
What is facilitated transport?
|
A form of movement across cell membranes through pores, channels, gates, and uniporters, that doesn't require energy but relies upon a concentration gradient.
|
|
|
What are gates, what is an example of one?
|
Gates are a form of facilitated transport, they are very specific and saturable. An example is the GLUT1 gated channel, specific to glucose. It's a 'uniporter' it only transports one molecule at a time. It works faster than passive diffusion and prevents glucose from coming in contact with the hydrophobic interior of the membrane.
** if it opens & closes it's a gate |
|
|
What are pores/channels? What is an example of one?
|
Integral membrane proteins which create holes in the membrane large enough for molecules to pass through. They are specific based on size but can be specific in other ways. A good example is the potassium resting channel.
|
|
|
How does the potassium resting channel work?
|
The mouth of the channel has four oxygen molecules positioned exactly as they are around potassium when it's in aqueous solution, this creates favorable binding of potassium to the channel, allowing it to pass through. Because Na+ is smaller and binds the oxygen in water closer, differently from K+ it can only bind two of the oxygen molecules present in the channel and it won't pass through because this binding is less favorable than the regular four bonds Na+ posses.
This is why the K+ channel is specific. The K+ channel also provides a charge to cells, an experiment involving an impermeable membrane with different amounts of salt but equal charge on both sides shows that if K+ resting channels are present potassium will move down it's concentration gradient, creating a positive charge on one side. |
|
|
What are voltage gated channels?
|
Channels that are opened only through a change in membrane potential (e.g. Ca2+ release channel on sarcoplasmic reticulum in muscle cells.
|
|
|
What is primary active transport?
|
It's a form of transport that requires energy from ATP to power pumps to create a favourable concentration gradient.
|
|
|
What is secondary active transport?
|
A form of transport that uses the concentration gradient created by primary active transport.
|
|
|
What do P class pumps pump?
|
H+, Na+, K+, Ca2+
|
|
|
What is Muscle Calcium ATPase>
|
A P class pump that pumps two Ca2+ per ATP out of the Cytosol into the SR lumen, removing it from the muscle cell.
|
|
|
What is Sodium/Potassium-ATPase?
|
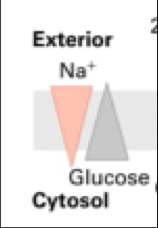
A P-class pump that pumps 3 Na+ out of the cytosol and 2 K+ into the cytosol for each ATP it uses.
This is an example of an antiporter. It results in high Na+ outside of the cell, high K+ in the cell. |
|
|
What do
F class, V class, and ABC-type pumps transport? |
F & V pump H+ only , they are found in mitochondria and chloroplasts.
ABC type pumps pump small molecules, ions, proteins lipids... 'ATP-Binding Cassette' |
|
|
What is a symporter?
|
A pump that moves more than one thing in the same direction.
|
|
|
What is a cotransporter?
|
An antiport or symport that moves two molecules, using the free energy of the ion gradient.
|
|
|
How does the sodium-glucose symporter work?
|
2Na+ moved down the gradient for 1 glucose against the gradient… Both sodium and glucose are moving into the cell. This happens because of sodium's concentration gradient AND an energy gradient as a result of potassium leaking out of the cell. The inside of the cell is negative and Na+ wants to move towards it.
|
|
|
How is glucose taken from the intestine to blood cells?
|
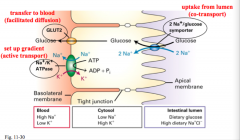
Through epithelial cells. Glucose brought into the cell from the intestinal lumen by the Na+/glucose symporter, and from there it can be transferred to the blood by GLUT2.
|
|
|
What does Glut2 do?
|
It moves glucose down it's concentration gradient across the basolateral membrane and into the blood.
|
|
|
What is signal transduction?
|
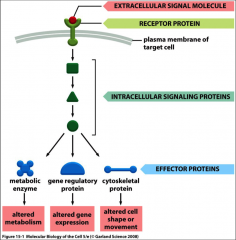
Conversion of one signal into another and it involves the external stimulus of growth factors, cytokines, hormones, the ECM, neurotransmitters, light, sound, etc.
|
|
|
What kind of receptors do steroids interact with?
|
They are good ligands for intercellular receptors, they are produced in glands, have a *hydrophobic nature, can cross the plasma membrane and bind to the receptors.
|
|
|
What two kinds of receptors are there?
How do they work? |
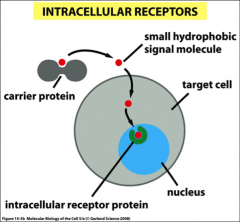
Cell surface & intracellular, they transmit signals by interacting with other effector proteins (causing them to change their function or activity) or second messengers (e.g. Ca2+, cAMP, cGMP, IP3, DAG, NO, etc.).
|
|
|
What is glucocorticoid?
|
An example of a steroid hormone (a potent anti-iflammatory and immunosupressive reagent,) the receptor is normally retained in the cytoplasm and bound to an inhibitor in the absence of glucocorticoid. The inhibitor stops the receptor from dimerizing AND from going into the nucleus. Glucocorticoid binds the ligand binding domain and displaces the inhibitor, this allows the receptor to move into the nucleus. Once in the nucleus, it forms a dimer allowing the DNA binding domain to bind to response elements. ‘Glucocorticoid response elements.’ This stimulates preinitiation complex assembly required for transcription (mRNA synthesis).
Activation domains are a portion of the protein that helps recruit in transcription factors, that bind to promoter region and activate transcription. GR = glucocorticoid receptor Inhibitor = HSP90 |
|
|
What is the nuclear-receptor superfamily?
|
Intracellular receptors that lipid soluble hormones bind to. These proteins have highly conserved DNA- and ligand-binding domains along with a variable region for specificity.
|
|
|
What are response elements?
|
The characteristic nucleotide sequences of the DNA sites that bind nuclear receptors.
|
|
|
What are effector proteins?
|
They can be metabolic enzymes, gene regulatory proteins, or cytoskeletal proteins.
|
|
|
What are the four forms of intercellular signalling?
|
1. Endocrine, signaling to distant cells.
2. Paracrine = signaling to nearby cells, ie. The signaling molecules released by a cell affect only those target cells in close proximity. (neurotransmitters) 3. Autocrine signaling = self signaling i.e. cells respond to substances that they themselves release. 4. Direct signaling, done by PM attached proteins, direct contact. |
|
|
How does termination of the cellular response to signal transduction occur?
|
Intracellular signaling molecules that inhibit receptor function are released and by removal of the extracellular signal.
|
|
|
How can extracellular signals rapidly/slowly alter cell behavior?
|
For immediate responses (milliseconds – minutes), receptor-bound extracellular signal molecules unleash an intracellular signalling pathway that leads to altered protein function, for slow responses receptor-bound extracellular signal molecules unleash an intracellular signalling pathway that leads to activated gene expression.
|
|
|
What are survival factors?
|
Extracellular signals that suppress apoptosis and convince the cell to persist as usual.
|
|
|
What do mitogenic signals do?
|
Combine with survival factors in order for the cell to grow and divide at an increased rate.
|
|
|
What is trophic factor withdrawal?
|
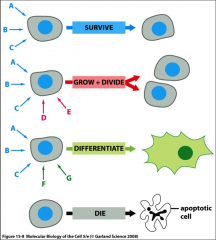
The removal or absence of basic survival factors without the survival signals present the cell will undergo apoptosis. Absence of signals. **The survival signals are present during grow & divide as well as differentiate.
|
|
|
What does binding specificity depend on?
|
Molecular complementarity between the interacting surfaces of a receptor (binding interface) and ligand (noncovalent forces such as electrostatic and hydrophobic interactions).
|
|
|
What is Kd? What is the equation for it?
|
The measure of affinity of a receptor to its ligand or the ligand concentration required to bind 50% of the cell surface receptors.
Kd = [R] [L]/[RL] Low Kd is good, a lower amount of ligand is needed for 50% of receptors to be bound. Low Kd = high affinity. |
|
|
What is a functional expression assay used for and how does it work?
|
To identify a cDNA encoding a cell surface receptor.
Transfect a cell line that doesn’t express receptor for ligand X with cDNA from a cell line that does express this. When you find a responsive cell line you can identify what part of the cDNA it has incorporated using PCR followed by sequencing. The receptor protein can be deduced from the cDNA sequence. |
|
|
How is protein activity regulated by a kinase?
|
Substrate protein is the target, it has amino acids with hydroxyl groups (serine, threonine, tyrosine) These are turned on by phosphorylation. Kinases put phosphate groups onto the hydroxyl groups, this is controlled by other amino acids on the protein that give specificity to the kinase binding.
|
|
|
What are G proteins?
|
One of the biggest signaling families, G proteins are another way to control protein activation. GTP binding is essential to G proteins. These exist in two forms Active = bound to GTP Inactive = bound to GDP.
|
|
|
What is GEF?
|
Guanine nucleotide exchange factor, helps reactivation of G proteins it displaces the GDP and causes the protein to bind GTP.
|
|
|
What is GAP?
|
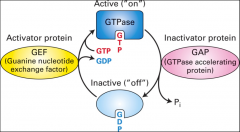
GTPase activating protein, it helps speed up GTP hydrolysis.
|
|
|
What are GPCR's?
|
G Protein-Coupled Receptors, the most numerous class of receptors in animal cells. A complex of trimeric G-proteins is usually coupled to these receptors, which have 7 membrane-spanning domains (α-helical regions). They also have membrane bound effector proteins and often second messengers.
|
|
|
What constitutes a trimeric G protein?
|
A complex of three protein subunits (Gα, Gβ, and Gγ).
Gα is lipid anchored at the cytoplasmic face of the plasma membrane, it binds GTP. It's 'physically linked to membrane.’ it can dissociate but Gβ, and Gγ are always associated. *it's function in signal transduction is coupling ligand-bound receptors to specific effector molecules. |
|
|
How is the effector protein activated by GPCR?
|
Following the ligand (hormone) binding to the GPCR, this induces a conformational change in the now active receptor and allows it to bind with the Gα subunit. The binding of the activated GPCR to the Gα subunit induces a conformational change in it and triggers the dissociation of GDP from its binding cleft. The later binding of a GTP molecule to the empty cleft on the Gα subunit causes the dissociation of the Gα and Gβ/γ subunits and the free and activated Gα-GTP binds to and activates an effector protein.
|
|
|
What is adenylyl cyclase?
|
A membrane associated effector protein.
|
|
|
What causes G-alpha to dissociate from it's effector?
|
The hydrolysis of GTP terminates their short-lived state and leads to the reassembly of the trimeric G protein complex.
|
|
|
What is FRET used for?
|
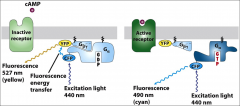
To show the receptor mediated dissociation of G-alpha and G-beta.
1) G-alpha fused to cyan fluorescent protein (CFP) 2) G-beta fused with yellow fluorescent protein (YFP). |
|
|
What are Muscarinic Acetylcholine Receptors?
|
Receptors found in the heart, associated with K+ channels.
An example of fast acting signal transduction. |
|
|
How does Acetylcholine interact with Muscarinic Acetylcholine Receptors?
|
Acetylcholine binds to its receptor, leads to opening of K+ channel and cellular hyperpolarization. (More posi outside of cell, more nega inside.) This slows the rate of heart muscle contraction.
**The difference in this is that when the acetylcholine receptor is activated by acetylcholine it promotes association of G-alpha subunit to the active receptor, however it’s the G-Beta/gamma not the G-alpha subunit that binds the potassium channel. (In this case the K+ channel is the effector). Here G-alpha is inhibitory because it inhibits G-beta/gamma from doing anything. |
|
|
What does Adenylyl Cyclase catalyze?
|
It catalyzes formation of cyclic AMP (cAMP), it's an enzyme that promotes very high levels of cAMP.
|
|
|
What activates/inhibits adenylyl cyclase?
|
The ligand receptor AND the alpha subunit determine whether there will be activation or inhibition. In many cases both of these pathways are present in a cell in competition.
The Gβ/γ subunit in both stimulatory and inhibitory G proteins is identical, while the Gα subunits and their corresponding receptors differ. |
|
|
How does cholera interact with adenylyl cyclase?
|
Cholera is the inappropriate activation of Adenylyl Cyclase, The pathogen secrets cholera toxin this binds to a receptor, is taken up into epithelial cells of the intestine, the toxin binds the G-alpha stimulatory unit, maintains its active state, resulting in mass activation of Adenylyl cyclase and massive increase in cAMP levels.
cAMP activates CFTR ion channels, Cl- is pumped out, Na+ follows it and madddd H2O loss occurs. Massive diarrhea, maybe death. |
|
|
What is whooping cough?
|
A result of the pertussis toxin. These get inside lung epithelial cells, bind G-alpha inhibitory, it’s an inhibitor of an inhibitor. Regularly low levels of G-alpha S are countered by G alpha I, when pertussis binds the G-alpha I it cant stop the low levels produced by G alpha S, this results in a steady increase of cAMP, binds CFTR, ions pump more rapidly, resulting in high levels of mucous, and water in the lungs.
|
|
|
What is epinephrine?
|
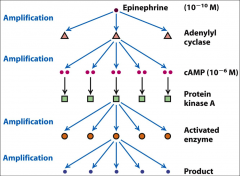
One of the major activators of adenylyl cyclase and thus increases the concentration of cAMP.
|
|
|
What is PKA?
|
Protein kinase A, a major mediator of cAMP. It's a tetramer (made up of four subunits).
There are two R (Regulatory) subunits, and two C (catalytic) subunits. |
|
|
What is cAMP
|
Cyclic AMP, a second messenger involved in the 'fight or flight' response, it's activated by adenylyl cyclase (which can be activated by epinephrine). It helps convert Glycogen → glucose, contract heart muscles, provide the energy needed to move to muscle tissue.
cAMP binds PKA. It's best effects are in the cytoplasm but it also has effects in the nucleus. |
|
|
What happens when cAMP binds PKA?
|
When cAMP binds one of the sites on the R subunits, this causes a higher affinity for the other domain by lowering the association constant for the other cAMP binding domain, when this happens that cAMP binds both binding domains on both R’s it causes the R subunits and the catalytic subunits to release. The C units can then go for and catalyze the phosphorylation of things.
|
|
|
What is an example of 'cooperative fashion?'
|
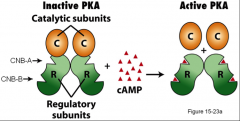
When cAMP binds to R, increasing the affinity for the next cAMP to bind R.
|
|
|
What is PKG?
|
Protein Kinase G – activated by cyclic GMP, opens cation channels in rod cells.
|
|
|
What is PKC?
|
Protein Kinase C - activated by 1,2 Diacylglycerol (DAG).
|
|
|
What is IP3?
|
Inositol triphosphate, (IP3) - Opens Ca2+ channels in endoplasmic reticulum.
|
|
|
What does PKA do?
|
Phosphorylates GPK activating it, phosphorylates GS inactivating it, this helps break down glycogen/stop glycogen synthesis.
As well it phosphorylates IP to prevent dephosphorylation of the two already mentioned. This occurs in the cytoplasm. |
|
|
What does GPK do?
|
(Glycogen phosphorylase kinase) Activates GP.
|
|
|
What does GP do?
|
(Glycogen phosphorylase), It catalyzes conversion of glycogen to glucose 1-phosphate.
|
|
|
What does PP do?
|
(Phosphoprotein phosphatase) It dephosphorylates things.
|
|
|
What does IP do?
|
It's the inhibitor of PP it binds PP to prevent dephosphorylation.
It is active when phosphorylated. |
|
|
What is CRE?
|
The cAMP response element, it's a nucleotide sequence found in found in genes regulated by PKA. 'Promoter regions inside the genome that respond to cAMP'
|
|
|
What is CREB?
|
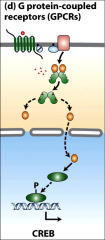
The CRE-binding protein it is activated by phosphorylation in the nucleus, particularly by PKA?
|
|
|
How does the CRE/CREB cycle work?
|
GPCR activates adenylyl cyclase, activates cAMP, promotes PKA C domain dissociation, catalytic domain goes into nucleus phosphorylates CREB, CREB dimerizes, binds (CRE) and CBP/P300 coactivator, CBP/P300 recruits transcriptional machinery to stimulate transcription of the various target genes controlled by the cAMP response element.
|
|
|
What is CBP/P300
|
A coactivator bound with CRE by CREB for transcription stimulation.
|
|
|
What is PLC?
|
Phospholipase C, it's an effector activated by G-alphaO or G-alphaQ. It cleaves PIP2 into DAG and IP3.
|
|
|
What does IP3 do?
|
When made from PIP2 by PLC it moves to the ER membrane and opens Ca2+ channels. The calcium translocates PKC to the plasma membrane.
It's inisitol 1,4,5 triphosphate, a carbohydrate associated with the phsophate DAG to make PIP2. *an important second messenger. |
|
|
What does DAG do?
|
Activates PKC, it's a second messenger that comes from PIP2, it's the lipid component of this, that remains in the membrane when IP3 is cleaved off.
|
|
|
What is PKC? What does it do and how is it activated?
|
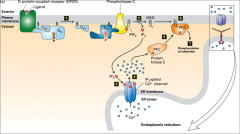
PKC is a protein kinase, it's moved to the plasma membrane by the Ca2+ released as a result of IP3 and it's activated by DAG.
It's main job is to phosphorylate substrates, including players that activate MAPK. |
|
|
What is PIP2?
|
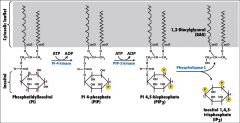
A version of Phosphatidylinositol (PI) -a lipid-linked carbohydrate- that has undergone many phosphorylation events, it is cleaved by the enzyme PLC, into IP3 and DAG.
|
|
|
What does Ca2+ nitric oxide (NO)-cGMP pathway role in?
|
The relaxation of arterial smooth muscle.
|
|
|
What is NO?
|
NO is a gas with a short half-life (2-30 seconds), it's readily diffusible to cross the PM.
It activates intracellular NO receptors that have guanylyl cyclase activity in nearby smooth muscle cells |
|
|
What does calmodulin do?
|
It activates NO synthase, Ca2+ binds to it, in the endothelial cell.
|
|
|
What is the NO receptor?
|
The nitrous oxide receptor, it has guanylyl cyclase activity and can convert GTP to cGMP.
|
|
|
What does cGMP do?
|
Activate PKG.
|
|
|
What does PKG do?
|
Phosphorylates different proteins/pathways such as actin and myosin.
|
|
|
What needs to happen for muscle relaxation to occur with NO?
|
Acetylcholine needs to bind it's GPCR, PLC must be activated, PLC must cleave PIP2, to IP3 and DAG, IP3 must diffuse to the ER and cause the release of Ca2+ which binds calmodulin, this in turn must activate NO synthase, nitrous oxide is made, comes in contact with NO receptors, these convert GTP to cGMP which activates PKG which phosphorylates protein pathways that lead to actin/myosin relaxation (muscle relaxation).
|
|
|
What is PDE?
|
Phosphodiesterase-5 - an inhibitor of cGMP. It converts cGMP to GMP, this is hyperactive.
|
|
|
How does Viagra work?
What are it's side effects? |
It works by inhibiting PDE (which converts cGMP to GMP) this increases cGMP & PKG activity and causes vasodilation of smooth muscle resulting in increased blood flow to the penis.
Side Effects: maintenance of erection, elevated blood pressure, blood circulation issues in other parts of the body, and cyanopsia (vision that is tinted blue) due to diminished enzyme activity sensitising the retinal rod cells, which are most sensitive to wavelengths of light that appear blue-green. |
|
|
What are RTK's?
|
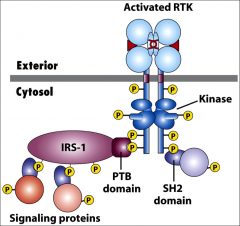
Receptor tyrosine kinases - membrane bound extracellular receptors with a single transmembrane α-helix.
Ligands include growth factors (NGF, PDGF, FGF, EGF) and insulin. They dimerize and phosphorylate tyrosines. |
|
|
How are RTK's activated?
|
Binding of a ligand (usually a growth factor) causes the receptor to dimerize and become active. It phosphorylates tyrosines ON ITSELF (on the cytoplasmic side) these 'phosphotyrosines' serve as docking sites which bind to adapter proteins (containing SH2 or PTB domains), and eventually activate Ras.
|
|
|
What is Ras?
|
A GTPase switch protein to signal further “downstream” kinases. It's a monomeric G protein, part of the GTPase superfamily, it's a lipid-anchored protein.
A major downstream effector of RTK signalling. Similar to G alpha but it can't hydrolyze GTPase very well. It has 'helper proteins' such as GEF & GAP. **It's final downstream effector is MAPK |
|
|
What do the phosphorylated domains on RTK's do?
|
They bind adapter proteins, bringing them close to the kinase part of the membrane bound protein.
|
|
|
What is SH2?
|
src homology 2 domain... A binding domain found in adapter proteins that recognizes phosphorylated tyrosine residues. (RTK's in this context.)
|
|
|
What is PTB?
|
Phosphotyrosine-binding domain. Found on multi-docking proteins, serve as docking sites for other signal transduction proteins, eg. IRS-1- Insulin receptor substrate protein.
|
|
|
What is src?
|
A gene that encodes a tyrosine kinase, in human cells this gene is found (c-src) without harm, but in chickens it's present in a viral form (v-src) that causes tumors. The difference between c-src and v-src lies in the carboxy terminus, the v src did not contain the entire gene, it left out the end of the gene that allowed for inhibitory phosphorylation & control of the gene expression.
Because of this the viral form is constitutively active. The protein is called an oncoprotein. tyrosine 527 is the what is missing in v src |
|
|
What is GAP?
|
A helper protein that helps Ras hydrolyze GTP (turns it to 'off' stage.) GEF is takes out the GDP so GTP can bind and GAP can work again.
|
|
|
What is RasD?
|
A mutation that makes the Ras gene turned on all the time, so it can't hydrolyze GTP when it's bound, mutation of glycine-12 which prevents binding GAPs (RasD protein).
This mutation is found in 20-30% of cancers. |
|
|
What is GRB?
|
Growth factor Receptor Bound protein, an adapter protein that binds to RTK's it has no enzymatic activity, it's merely a bridge.
It recognizes phosphorylated domains with it's SH2 domain, the SH3 domain is exposed and can bind sos (a GEF) which binds Ras proteins. |
|
|
What is an SH3 domain?
|
A domain on adapter proteins that has a high affinity for prolein residues.
|
|
|
What is the function of sos?
|
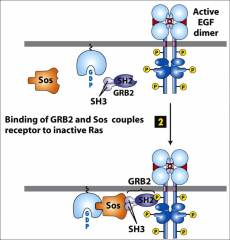
To bind Ras, it has GEF activity when bound properly to everything. It helps displace GDP from Ras.
|
|
|
What is MAPK?
|
Mitogen Activated Protein Kinase. It's activated by MEK phosphorylation, it translocates to the nucleus and activates many transcription factors that control cell cycle, cell growth and cell differentiation.
*It's other name is ERK (extracellular signal-regulated kinase.) |
|
|
What is EGF?
|
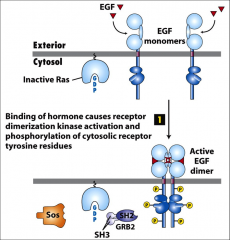
External growth factor? A ligand that binds EGF receptors. EGFR's are RTK's that dimerize and in turn activate Ras. It promotes the MAPK pathway.
|
|
|
What is RAF and how is it activated?
|
A cytosolic protein, a kinase that is inactive when it's floating around, when Ras is active it can bind Raf, a bit of the inhibitory regulation (protein 14-3-3) is popped off of Raf, Ras has it's GTP hydrolyzed and Raf becomes active. It phosphorylates serine & threonine residues on MEK.
|
|
|
What is the Ras--> MapK cascade?
|
Ras activates Raf activates MEK activates MAPK
|
|
|
What is MEK?
|
A kinase with multiple abilities, it is phosphorylated by Raf. It phosphorylates threonine tyrosine and serine.
It phosphorylates and activates MAPK. |
|
|
What is MAPK
MAPKK MAPKKK |
Mitogen Activated Protein Kinase,
MEK Raf. |
|
|
How does src play a roll in cancer?
|
It can often phosphorylate downstream parts of the MAPK pathways.
|
|
|
What is the Her2 receptor?
|
A variation in the EGF receptor, a mutation in it can cause spontaneous dimerization in the absence of the ligand.
(associated with neuroblastoma). Her2 can bind hetero or homo dimers, it can form a dimeric complex with a different EGF (Like her 1). ~25% breast cancer patients have increased HER2 expression A mutation that alters a single amino acid (valine to glutamine) in the transmembrane region of the Her2 receptor causes dimerization (and thus, activation) of the receptor, even in the absence of the normal EGF-related ligand, making the Neu oncoprotein (dimer of the Her2 receptor) |
|
|
What is Herceptin?
|
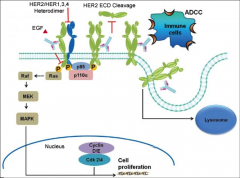
The only approved HER2 therapy designed to bind to HER2+ tumor cells and flag them for destruction by immune system. It also blocks down stream HER2 signaling to inhibit proliferation of cells.
|
|
|
What leads to constitutive activation of ErbB oncoprotein’s kinase activity?
|
A deletion that causes the loss of the extracellular ligand-binding domain in the EGF receptor
|
|
|
What are the Human Epidermal Growth Factor Receptors?
|
Four receptor tyrosine kinases, Her1-4.
|
|
|
What are apoptosis and necrosis?
|
Apoptosis (also known as programmed cell death) is a key process that ensures normal embryonic development and proper tissue homeostasis in adult animals. Programmed cell death is an active cellular process which requires energy as opposed to necrosis (the passive form of cell death) where cells simply swell up and die.
|
|
|
How does apoptosis occur?
|
Apoptotic cells tend to shrink somewhat as well, and blebs (irregular bulges in plasma membrane) start to become visible. As this progresses, the blebbing continues and the nucleus actually breaks down in smaller fragments known as apoptotic bodies, which are recognized and engulfed whole by phagocytic cells in order to minimize cellular debris produced.
|
|
|
What is ICAD?
|
A DNAase that participates in apoptosis , it cleaves DNA in between nucleosomes creating 200 bp fragments.
|
|
|
What are ced genes?
|
Genes that program for cell death (apoptosis.)
In worms with mutants in Ced 3 and Ced 4 there were more cells than there should have been, 'undead cells.' |
|
|
What is Ced3?
|
A gene that codes for apoptosis. An 'effector caspase.'
It assists with; DNA fragmentation (by allowing for DNAase ICAD activation), fragmentation of nucleus (by cleaving nuclear lamins), membrane blebbing (through cytoskeletal disruption of important proteins), and fragmentation of Golgi (by influencing Golgi matrix proteins). |
|
|
What is the Apoptosome?
|
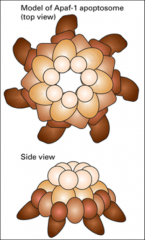
1.4 megadalton wheel of death
It's a heptamer of Apaf-1 (apoptotic protease activating factor 1), a protein that assembles in response to apoptosis signals and serves as an activation machine for initiator and effector caspases. |
|
|
What are caspases?
|
Cysteine-dependent aspartate-directed proteases, they play a key role in apoptosis. There are two forms of caspases present: initiator caspases (that cleave inactive pro-forms of effector caspases, thereby activating them) and effector/executioner caspases (that cleave other protein substrates within the cell, to trigger the apoptotic process).
|
|
|
What is Apaf-1?
|
Apoptotic protease activating factor 1, it normally exists inactive in the cytosol, but when it's bound by cytochrome C it forms the wheel of death (apoptosome.) It becomes a heptamer when it forms the wheel of death and it recruits inactive Caspase 9, which dimerizes, becoming active.
|
|
|
What does caspase 9 do?
|
It's an initiator caspase that cleaves inactive forms of downstream effector caspases (like ced-3), activating them for the process of apoptosis to be underway.
|
|
|
What is Bcl-2?
|
A pro-survival (antiapoptotic) protein that inhibits apoptosis by binding Bax & Bak and whose high expression was found in human B-cell lymphoma.
|
|
|
What are the three types of Bcl Family proteins?
|
pro-survival members (have extra BH4 domain; form channels in mitochondrial outer membrane), pro-apoptotic members (missing BH4 domain) and BH3-only proteins (regulate activity of the other two classes, particularly Bcl-2 and Bax/Bak).
**Blc-2 is prosurvival. |
|
|
What are Bax and Bak?
|
examples of pro apoptotic proteins.
|
|
|
What are Bim, Puma, and Bad?
|
BH3-only proteins that control pro apoptotic and pro survival proteins. Bim & Puma bind Bak & Bax and cause them to form oligomeric channels in the outer mitochondrial membrane.
|
|
|
What is the ATM pathway?
|
ATM is a kinase that can phosphorylate and active p53, this leads to increased expression of PUMA which interacts with antiapoptotic Bcl-2 family members and frees Bak/Bax to form oligomeric channels, that allow chytochrome C in where it binds to the adapter protein Apaf-1, promoting caspase 9 activation that initiates the apoptotic cascade and leads to cell death.
|
|
|
What is PUMA?
|
A BH3-only protein. Puma interacts with pro-survival Bcl-2 family members, and frees Bax and/or Bak to form oligomeric channels.
It's activated by p53 and leads to apoptosis. |
|
|
What is TrKA?
|
Receptor protein tyrosine kinase - a kinase activated by trophic factors that repress the apoptotic pathway.
|
|
|
What is PKB?
|
PI-3 kinase/protein kinase B it phosphorylates Bad (BH3-only protein) and phosphorylated Bad then forms a complex with the 14-3-3 protein. With Bad sequestered, it is unable to bind to BcI-2. In the absence of trophic factors, unphosphorylated Bad binds to Bcl-2, releasing Bax and Bak and allowing them to form the oligomeric channel.
|
|
|
What is Bad?
|
A BH3 only protein, when unphosphorylated it binds Bcl2, releasing Bax and Bak, leading to oligomeric channels & apoptosis.
When it's phosphorylated it forms a complex with 14-3-3 (inhibitor protein) and it's inactive, it apoptosis is prevented. When trophic factors aren't present Bad ISNT phosphorylated and Bad is free to bind Bcl-2. |
|
|
How is apoptosis regulated?
|
When Bim is present because of disruption of integrin signalling, or PUMA released from the nucleus as the result of DNA damage and p53, Bcl-2 (the inhibitor of bak or bax) is bound, releasing bak/bax causing the formation of channels in the mitochondrial membrane that allow the release of cyctochrome C, ctyochrome C binds Apaf-1 allowing for the formation of the 'wheel of death' and the recruitment of (pro)caspase 9 which dimerizes, becomes caspase 9, cleaves procaspase 3 to caspase 3, this goes forth and causes apoptosis.
|
|
|
What is fasL, how does the extrinsic apoptosis pathways work? ?
|
The fas ligand, a positive acting death signal. When it binds to the receptor on another cell it recruits the Fas-Associated Death Domain (FADD) and the dimerization and activation of caspase-8 Active caspase-8 then cleaves and activates caspase-3, caspase-6, and caspase-7, which then cleave vital cellular substrates and induce cell death. Cleavage of the BH3-only protein BH3-Interacting-Domain Death Agonist (BID) by caspase-8 generates a t-BID fragment that binds to Bcl-2 on the mitochondrial outer membrane, leading to the release of cytochrome c, into the cytosol and activation of the intrinsic apoptosis pathway.
|
|
|
hurp durp apoptosis...
|
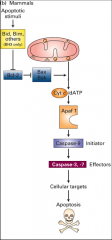
|
|
|
What is a microtubule?
|
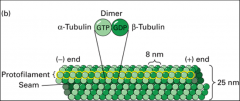
A repeating structure (polymer) of α and β tubulin monomers. Together, an α and β tubulin form an αβ dimer as the basic microtubule subunit. Because they conjugate into a dimer, α and β tubulin are almost never found separately. Instead they stretch end to end in a dimeric form, creating a protofilament (striated chain of tubulin dimers) that has polarity (one end α and one end β). It takes 13 protofilaments to form the hollow tube structure of a microtubule.
|
|
|
How does a protofilament grow?
|
Hydrolysis of the Beta end of the microtubule chain,
|
|
|
What is the centrosome?
|
The major microtubule-organizing center of (MTOC) of animal cells (not in plant cells); they contain mother and daughter centrioles which are small barrel-shaped structures made of triplet microtubules that lie 90° to each other.
-In the MTOC called the centrosome, γ-tubulin associates with other proteins to form the γ-tubulin ring complex and provides the nucleating site for growing microtubules in the centrosome of an animal cell. To this γ-tubulin ring complex, α and β dimers are added, extending in a positive (plus end) direction. |
|
|
What is the pericentriolar matrix?
|
A matrix of protein (γ-tubulin, augmin complex, etc.) outside of the centrioles that allows for the polymerization of microtubules.
|
|
|
What is the MTOC?
|
The microtubule organizing centre. Functions to nucleate (act as a starting point for) the assembly of microtubules. Examples of MTOCs are present in spindle poles (mitotic apparatus), cilia and basal bodies. The minus end is associated with the α-tubulin end (towards the MTOC), while the plus end is always associated with the β-tubulin end (grows away from the MTOC).
|
|
|
What are the four steps of the cycle whereby MT's are always changing?
|
assembly (expansion of microtubules), catastrophe (the start of depolymerisation), disassembly (contraction of microtubules, and rescue (the start of polymerization or reassembly).
|
|
|
What is the GTP-β-tubulin cap
|
Assembly or growth of the microtubule requires the constant addition of GTP-β-tubulin caps. The hydrolysis of a GTP-β-tubulin cap to a then added GDP-β-tubulin cap causes instability on the 5’ end
Dynamic instability depends on the presence or absence of a GTP-β-tubulin cap. |
|
|
What does Gamma tubulin do?
|
Nucleates polymerization of MT's with it's ring complex.
It binds the minus end. |
|
|
What is colchicine?
|
An MT disrupting drug, it causes depolymerization.
|
|
|
What is taxol?
|
An MT disrupting drug, it causes stabilization of MT's.
Important in cancer treatment. |
|
|
What are MAPs?
|
MT Associated Proteins - used to alter the stability of microtubules. They essentially bundle microtubules closer together and can be regulated by phosphorylation promoting disassembly (like CDK in the cell cycle), important in mitosis. The axons in nerve cells possess huge stable microtubules (like in the long sciatic nerve) and are coated with MAPs for attaining optimal stability.
|
|
|
What is Tau?
|
A type of MAP it contains a binding domain and a projection domain and is used to stabilize microtubules.
|
|
|
What is MAP2?
|
A type of MAP it contains a binding domain and a projection domain and is used to stabilize microtubules. Map2 have projection domains that are packed further apart than those observed in Tau and this projection pattern affects functions (due to spacing) like transport.
|
|
|
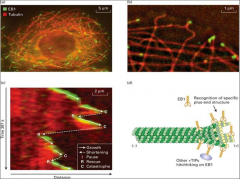
What are +tips?
|
Special MAPS - Plus-end tracking proteins - they are associated with the plus end of microtubules.
|
|
|
What is EB1?
|
a +TIP that map bring other molecules to the plus end. EB1 stabilizes the plus end, but moves along the seam (probably taking other hitchhiking proteins with it) to the plus end. Recall that the stability of the microtubule is related to the plus end tip (plus end tip present = growth, plus end tip absent = disassembly). EB1 proteins usually promote microtubule dynamics and growth, and suppress catastrophes in cells.
|
|
|
What does stathmin do?
|
Promotes the destabilization of MT's
It's a +TIP that map bring other molecules to the plus end. EB1 stabilizes the plus end, but moves along the seam (probably taking other hitchhiking proteins with it) to the plus end. Recall that the stability of the microtubule is related to the plus end tip (plus end tip present = growth, plus end tip absent = disassembly). EB1 proteins usually promote microtubule dynamics and growth, and suppress catastrophes in cells. |
|
|
What is Kinesin 13? What does it do?
|
It promotes the destabilization of MT's . Kinesin-13 family members have the motor domain in the middle of their heavy chains (only head domains) and do not have motor activity, but they do destabilize/disassemble microtubule ends (using ATP to remove dimers from ends).
|
|
|
What is Kinesin?
|
The plus-end directed (ATP-dependent) motor protein of microtubules .
The common types of kinesin have 2 heavy cains (head, linker region and stalk) and 2 light chains (variable domain). The heavy chains possess ATPase activity and microtubule-binding ability (quite conserved), while the light chains recognize different types of cargo (thus the variability). |
|
|
What is Kinesin-1?
|
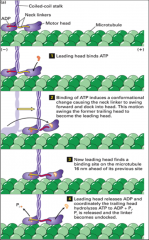
It's conventional in nature to the usual description of the kinesin motor protein as it binds different cargo and moves to the plus-end using ATP.
Has two heavy chains & two light. ATP is hydrolysed as each head moves 16 nm. |
|
|
What is Kinesin-5?
|
It has four heavy chains (no light chains) assembled in a bipolar manner to interact with two antiparallel microtubules and also move toward the plus-end (through a process known as sliding).
|
|
|
What is Dynein?
|
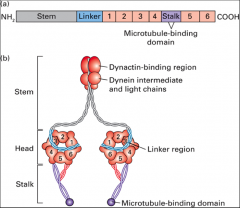
A minus-end directed motor protein and is involved in retrograde transport. The dynein heavy chain heads have ATPase activity and the stalk domain.
|
|
|
What does the dynactin hetero complex do?
|
Recognizes and binds cargo to dynein. Dynactin links dynein to cargo and regulates its activity.
|
|
|
What is the axoneme?
|
a microtubule arrangement comprised of a 9 + 2 array (nine, linked, outer doublet microtubules along with 2 inner, core singlets) which forms the underlying structure of cilia and flagella. Each outer A tubule of the 9 outer doublets has a radial spoke head that links them with the inner singlets (it is unclear what the function of the singlets is, but much more stable than conventional singlets). The A tubule of one doublet is linked to the B tubule of another by nexin and each A tubule has attached two dynein arms: one facing the singlet pair (inner-arm dynein) and one facing the plasma membrane (outer-arm dynein).
|
|
|
What is a basal body?
|
A structure built around nine linked triplet microtubules (A, B and C). The continuous axoneme is attached to a sturdy basal body in the cell. The basal body itself is similar in design and purpose to centrioles (both MTOCs) and its C tubules are limited (not the case for A and B tubule) by the transitional zone (region between the basal body and the axoneme).
|
|
|
What does nexin do?
|
The A tubule of one doublet is linked to the B tubule of another by nexin, nexins allow cilia/flagella to bend instead of just letting MT's slide past eachother.
|
|
|
What is the non-motile primary cilium?
|
The primary cilium is a sensory organelle, acting as the cell's "antenna" by detecting extracellular signals (e.g. sense of smell).
|
|
|
What are the six steps of karyokinesis?
|
Prophase (the breakdown of the interphase microtubule display and its replacement by mitotic asters), prometaphase (breakdown of the nuclear membrane and chromosomes are captured by the mitotic apparatus), metaphase (chromosomes are aligned at the metaphase plate), anaphase (A: chromosomes move to the poles; B: separation of spindle poles), telophase (assembly of contractile ring), and cytokinesis (reformation of interphase microtubule array).
|
|
|
What does XMAP215 do?
|
Suppresses catastrophes induced by kinesin-13. During mitosis it's greatly inhibited.
|
|
|
What makes up the mitotic appartus?
|
Astral microtubules project toward the cortex and are linked to it. Kinetochore microtubules are connected to chromosomes. Polar microtubules project toward the cell center with their distal plus-ends overlapping (missing the chromosomes). The spindle pole and associated microtubules is also known as a mitotic aster.
|
|
|
What is the centromere?
|
The constricted DNA sequence required for proper segregation of chromosomes during mitosis and meiosis and is the region of mitotic chromosomes where the kinetochore forms. It is the outer kinetochore and inner kinetochore proteins that constitute the kinetochore and mediate the attachment of chromosomes to microtubules. Microtubules are captured away from the outer kinetochore. This is so the microtubule plus-end can be assembled and disassembled easily.
|
|
|
What happens during congression?
|
Bi-oriented chromosomes then move to a central point between the spindle poles. Itinvolves bidirectional oscillations of chromosomes, with one set of kinetochore microtubules shortening on one side of the chromosomes and the other set lengthening on the other. On the shortening side, a kinesin-13 protein stimulates microtubule disassembly and a dynein-dynactin complex moves the chromosome toward the pole. On the side with lengthening microtubules, kinesin-7 protein holds on to the growing microtubule.
|
|
|
What is Ndc80?
|
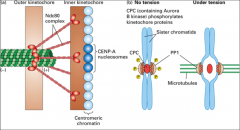
A kinetochore protein that when phosphorylated leads to microtubule instability. Under no tension, the phosphorylation of Ndc80 proteins at the kinetochore results in weak microtubule interactions with the kinetochore.
|
|
|
What is actin?
|
Actin monomers, known as G-actin, are globular in nature as they are divided by a central ATP-binding cleft. An actin polymer, known as F-actin, is essentially two chains of G-actin monomers configured into a double-helix. The ATP-binding cleft of every chain subunit is oriented towards the same end of the filament. The end of a filament with an exposed binding cleft is the minus-end, while the opposite end is the plus-end.
|
|
|
What does actin do?
|
Actin is very much involved in directing cell shape and migration. Common actin-based structures such as epilethial cells, migrating cells (e.g. erythrocytes) and muscle cells must all perform functions that are ultimately reliant on the presence of actin.
|
|
|
What do Anaphase A & B require?
|
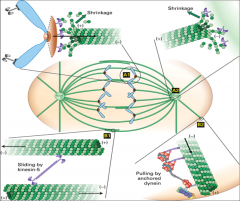
Anaphase A requires MT shortening
Anaphase B (pole separation) requires motors. |
|
|
What are the Cc's of actin's ends?
|
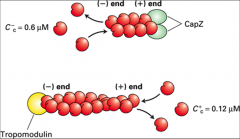
The critical concentration of the minus-end of actin is 0.60μM, the +end is 0.12μM, leads to 'treadmilling.'
|
|
|
What does profilin do?
|
Promotes actin polymerization cycle, profilin binds ADP-G-actin and catalyzes the exchange of ADP for ATP. The ATP-G-actin-profilin complex can deliver actin to the plus- end of a filament with dissociation and recycling of profilin.
|
|
|
What does cofilin do?
|
Enhances depolymerization cycle, cofilin binds preferentially to filaments containing ADP-actin, inducing them to fragment and thus enhancing depolymerization by making more filament ends.
|
|
|
What does thymosin do?
|
Sequesters actin cycle, G-actin available from the actin-profilin equilibrium is bound by thymosin, sequestering it from polymerization. As the free G-actin concentration is lowered by polymerization, G-actin-thymosin dissociates to make free G-actin available for association with profilin and further polymerization. Most of the free G-actin in the cell is bound by thymosin.
|
|
|
What is capZ?
|
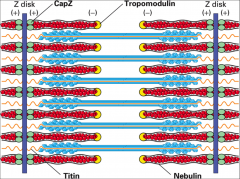
A capping protein that blocks the plus-end, which is where microfilaments normally grow, so its function is to limit actin dynamics to the minus-end (prevent assembly).
|
|
|
What does tropomodulin do?
|
Blocks minus-ends of actin, where microfilament disassembly normally occurs, thus the major function of tropomodulin is to stabilize filaments (prevent disassembly).
|
|
|
What are formins?
|
They have a domain called FH2 that can form a dimer and nucleate filament assembly. The dimer binds two actin subunits and, by rocking back and forth, can allow the insertion of additional subunits between the FH2 domain and the plus-end of the growing filament. The FH2 domain protects the plus-end from being capped by capping proteins. The inactive formin is activated by binding its Rho-binding domain (RBD) to membrane-bound active Rho-GTPase, resulting in exposure of the formin's FH2 domain, which can then nucleate the assembly of a new filament.
|
|
|
What is Arp 2/3?
|
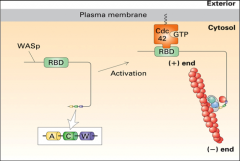
A complex recruited by NPF's to cause actin branching, it's activated by WASp which is activated by cdc 42??
|
|
|
What is fimbrin?
|
A protein found in microvilli that builds BUNDLES of filaments all having the same polarity.
|
|
|
What does spectrin do?
|
Makes networks of actin under the plasma membrane.
|
|
|
What does filamin do?
|
It stabilizes cross-links between filaments in a meshwork found in the leading edge of motile cells) help to form complex networks.
|
|
|
What does dystrophin do?
|
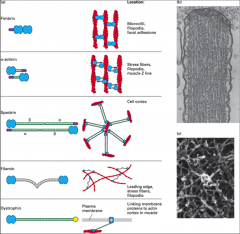
Links plasma membrane proteins to the actin cortex in muscle cells.
|
|
|
What does ezrin do?
|
Links actin filaments laterally to the plasma membrane in surface structures such as microvilli, where the attachment can be direct or indirect.
|
|
|
What is WASp?
|
An NPF that activates ARP2/3
|
|
|
What is the chain of transfer for muscle cells?
|
Contraction of actin and myosin -> Dystrophin -> Glycoprotein complex (transmembrane protein) -> Extracellular matrix -> Tendon -> Bone.
|
|
|
What is myosin?
|
Myosin is known as the motor protein of actin, in which the most abundant class is MII myosin, found in muscle cells. Myosin has an ATPase head (with an actin binding site), light chains (essential and regulatory that comprise the neck), and a tail that binds cargo (heavy chains). Calcium ions are very important for the function of myosin. The S1 myosin (head domains) is used to decorate the F-actin and help identify the minus-end.
Walks towards the + end |
|
|
What is myosin 1?
|
Myosin I consists of a head domain with a variable number of light chains associated with the neck domain. Members of the myosin I class are the only myosins to have a single head domain and associate directly with membranes through lipid interactions (like in endocytosis).
|
|
|
What is Myosin V?
|
Proteins have two head domains and six light chains per neck. They bind to specific receptors on organelles, which they transport (also involved in transport of endocytic vesicles).
It has a step size of 36 nm, yet each head moves in 72-nm steps, so it moves hand over hand. . In medium-sized yeast buds myosin V proteins transport secretory vesicles down actin cables nucleated by formins located at the bud tip and bud neck. Myosin V proteins are also used to segregate organelles, such as the vacuole (yeast equivalent of a lysosome), peroxisomes, endoplasmic reticulum (ER), trans-Golgi network (TGN), and even selected mRNAs into the bud. Myosin V also binds the ends of cytoplasmic microtubules to orient the nucleus in preparation for mitosis. |
|
|
What is a myofibril?
|
Thousands of repeating contractile structures called sarcomeres. Sarcomeres are repeating structures of stable thick filaments, found in skeletal muscle.
|
|
|
What are A & I bands?
|
On either side of the Z-disks are the lightly stained I bands, composed entirely of actin thin filaments (made of actin). These thin filaments extend from both sides of the Z disk to interdigitate with the dark-stained myosin thick filaments in the A band. Important to remember are that the A band is where thick filaments are (no actin) and the I band is where thin filaments are (no myosin II).
I bands decrease in length during the contracted state. |
|
|
What happens in muscles cells in the presence of Ca2+?
|
In the presence of Ca2+, the ions bind to troponin (already bound to tropomyosin) and causes tropomyosin to move its position on actin and reveal the myosin-binding domains.
|
|
|
What happens in smooth muscle contraction?
|
In the myosin phosphorylation mechanism for regulating smooth muscle contraction, phosphorylation of the myosin regulatory light chain activates contraction. At low Ca2+ concentrations, the regulatory light chain is not phosphorylated and the myosin adopts a folded conformation. When the Ca2+ level rises, it binds calmodulin (CaM), which undergoes a conformational change (CaM*).
|
|
|
What are stress fibers?
|
Contractile stress fibres associated with cell adhesion are long actin fibres which are interacting with myosin and other regulatory molecules; they are involved in cell movement as well. The meshwork of actin filaments in the leading edge advances the cell forward.
These fibers are contractile. |
|
|
What are focal adhesions?
|
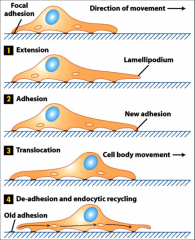
The structure of focal adhesions involves the attachment of the ends of stress fibres through integrins to the underlying extracellular matrix.
|
|
|
What does Rac do?
|
Induces the formation of a multitude of actin networks (for creating lamellipodiae).
|
|
|
What does Rho do?
|
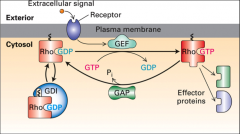
induces abundant contractile stress fibres.
|
|
|
What does cdc 42 do?
|
Induces the formation of lots of actin bundles (for creating filopodiae).
|
|
|
What are Intermediate filaments?
|
The simplest unit of intermediate filaments are relatively large proteins with polarity (one C-terminus end and one N-terminus end) and head, rod and tail domains. Two of these proteins form a dimer, The building block is a tetramer (two dimers), which doesn’t have any polarity (ends are the same). Tetramers then come together (polymerize without requiring energy sources like ATP and GTP) to form a protofilament.
Intermediate filament (IF) subunits don’t bind any nucleotides. They have great tensile strength, are assembled onto pre-existing filaments, are less dynamic than other cytoskeletal structures, do not have polarity or motor proteins, and assist in the integrity of cells and tissue. |
|
|
Where are keratins found?
|
Found in skin cells produced in epithelial cells.
|
|
|
Where is desmin found?
|
Muscle cells, it helps support the sarcomere.
|
|
|
Where is vimentin found?
|
Mesenchymal cells (poorly-differentiated migrating cell; e.g. fibroblasts).
|
|
|
What does lamin do?
|
Supports the nucleus, found in almost all cells. It's disassembled during mitosis.
The phosphorylation of (a serine residue on the N-terminal domain of) nuclear lamins by a mitotic cyclin-dependent kinase (that becomes active early in prophase of mitosis) induces the disassembly of intact intermediate filaments and prevents their reassembly. |
|
|
What are IFAPs?
|
Intermediate Filament-Associated Proteins (IFAPs) are a group of proteins that co-purify with intermediate filaments.
|
|
|
What is plectin?
|
An example of a plakin that works to cross-link IF proteins like vimentin with microtubules to achieve optimal tensile strength.
|
|
|
What is Lap2?
|
A type of IFAP known as lamin-associated polypeptides, links lamins to the nuclear lamina?
|
|
|
What are desmosomes?
|
Junctions between epithelial cells that provide stability to a tissue.
cadherins bound to intermediate filaments |
|
|
What are hemidesmosomes?
|
They are located at regions of the plasma membrane where intermediate filaments are linked to the extracellular matrix
|
|
|
What are junctions?
|
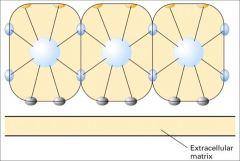
Patches in between cells and and the ECM that are linked to the cytoskeleton, and to intermediate filaments in particular.
*the blue are desmosomes. |
|
|
What are CAMS?
|
Cell adhesion molecules.
provide cell strength by gluing the cells to something else. These interactions can be homophilic (involve the same molecule on both the cells and must be between cells) or heterophilic (involve a molecule binding to a different molecule and can be between cells or between a cell and the ECM). There are four types of CAMs: Cadherins and IG superfamily (only homophilic interactions), and integrins and selectins (only heterophilic interactions). |
|
|
What are Gap junctions?
|
A way for cells to communicate, made of 6 connexins = a connexon, regulated by Ca2+ .
|
|
|
What are tight junctions?
|
A way to glue plasma membranes of neighbouring cells together and allow the formation of a barrier.
3 types of proteins = occludin, claudin & JAM |
|
|
What are cadherins?
|
A type of CAM with only homo interactions, Ca2+ dependent.
They are transmembrane molecules (part of the protein is in the cytoplasm and in the ECM) and the cytoplasmic domain is responsible for binding to the cytoskeleton in order to provide strength to the adhesion. |
|
|
What are N-cams?
|
Neural Cell Adhesion Molecules (N-CAMs) are members of the IG-superfamily, which constitute the other family of cadherins partaking in homophilic interactions. The IG superfamily is Ca2+-independent and there are many different types of cell adhesion molecules (e.g. I-CAM).
|
|
|
What are selectins?
|
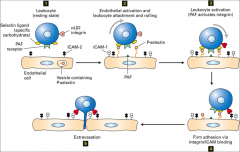
cell-surface molecules that involve the heterophilic binding to sugars
|

