![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
322 Cards in this Set
- Front
- Back
|
Explain a holosystolic murmur in a patient with known pulmonary hypertension |
Most likely due to tricuspid regurgitation due to pressure build up in the lungs, forcing the right ventricle to have to contract harder, and blood flow into the lungs not overcoming the pressure already there |
|
|
Cigarette smoking is most commonly associated with which types of lung cancer? |
Squamous cell and small cell
|
|
|
Pseudohemoptysis |
From places other than lungs/respiratory tract. Most commonly from GI due to trachea/esophageal fistula |
|
|
Which serum markers are most commonly associated with Granulomatosis with polyangiitis (Wegener)? |
PR3-ANCA/c-ANCA (Anti-proteinase 3)
Focal necrotizing vasculitis, necrotizing granulomas in lung/upper airway and necrotizing glomerulonephritis (URT, LRT, & Renal). Can cause alveolar hemorrhage |
|
|
Which serum marker is most associated with microscopic polyangiitis? |
P-ANCA (anti-myeloperoxidase)
Like granulomatosis with polyangiitis (wegeners), but doesn't have granulomas or nasopharyngeal involvement |
|
|
Churg Struass |
Granulomatous necrotizing vasculitis, eosinophilia (heart and respiratory tract).
-asthma, peripheral neuropathy, pANCA , and Increased IgE |
|
|
How do you tell if a patient is a responder after a prostaglandin I2 infusion to test relief of symptoms of pulmonary hypertension? |
If pulmonary artery pressure drops at least 10mmHg, and remains below 40 mmHg after dropping |
|
|
Burden of prostacyclin infusion pump |
-Continuous IV infusion if pt fails to response to vasodilator test to treat pulmonary hypertension. It is a permanently implanted central venous catheter using a portable infusion pump |
|
|
COPD pulmonary function test findings |
Increased Reserve volume, decreased forced vital capacity (airways close prematurely at high lung volumes).
Decreased FEV1, FVC, decreased ratio, and VQ mismatch present |
|
|
Pathophys of Emphysema |
Destruction of elastic tissue, decreased elasticity (recoil), increased compliance.
Decreased DLCO due to destruction of alveolar walls |
|
|
Causes of Emphysema |
-Cigarettes- Chemotaxis to neutrophils and macro--> release free radicals-->inactivates Alpha1-antitrypsin and glutathione (free radicals).
-AAT deficiency--> protease inhibitor that protects tissue from neutrophil elastase |
|
|
What causes the decrease in elastic fibers in emphysema? |
Excess elastase (destruction of elastic tissues) Excess free radicals |
|
|
Where is the damage due to emphysema from smoking most commonly found? |
Centriancinar (distal alveoli spared) |
|
|
Where is the damage due to emphysema from ATT deficiency most commonly found? |
Panacinar Lower lobe |
|
|
Pathophys of chronic bronchitis |
Inflammation, FIBROSIS,Hypertrophy/hyperplasia of mucus secreting glands in bronchi
Reid index (thickness of gland later/total bronchial wall thickness >50% |
|
|
What is the most common acid base disturbance of a person with chronic bronchitis? |
Chronic respiratory acidosis because CO2 is not getting blown off.
They have late dyspnea |
|
|
Define pulmonary hypertension |
mean pulmonary arterial pressure greater than/equal to 25 mmHg at rest |
|
|
Cor pulmonale |
Right ventricular hypertrophy/dilation and or imparted function due to pulmonary HTN |
|
|
Pathologic findings in pulmonary hypertension |
Arteriosclerosis, MEDIAL hypertrophy, and initial fibrosis of pulmonary arteries |
|
|
Genetic link to group 1 pulmonary hypertension |
Inactivation mutation in BMPR2 gene (normally inhibits vascular smooth muscle proliferation
Is a cell surface prtoeint in the TGF-Beta family and requires the two hit hypothesis |
|
|
Group 2 of pulmonary hypertension |
Congenital/acquired heart diseases
(mitral stenosis-->increased resistance--> increased LA pressure-->transmitted to pulmonary circuit) |
|
|
Group 3 of pulmonary hypertension |
Lung disease/hypoxemia.
--Due to COPD (destruction of parenchyma---> increased pulmonary resistance) --ILD --TX: GiveO2 |
|
|
Risk Factors for Female urinary incontinence |
Age, Childbearing, obesity, other urinary symptoms, functional impairment |
|
|
Age related changed to lower urinary tract |
1. Increased involuntary detrusor contractions. 2. Decreased detrusor contractility 3. Decreased urethral closure pressure 4. Atrophy of urethral mucosal epithelium (due to decreased estrogen post-menopause--> can lead to decreased urethral mucosal seal, loss of compliance, irritation 5. Changes in diurnal pattern of fluid excretion |
|
|
Urge incontinence |
-Sx: leakage +Urgency - Few drops--> soaked underwear -common triggers: running water, hand washing, cold weather -Believed to be partly caused by detrusor overactivity -more common in older women |
|
|
Stress incontinence |
Sx: Leakage with effort, exertion, sneezing, coughing, laughing, increased abdominal pressure Most common type in younger women Can coexist with urge =mixed |
|
|
Overflow Incontinence |
Sx: involuntary, continuous leakage, incomplete bladder filling -Caused by decreased detrusor contractility of bladder outlet obstruction (rare in women, most likely due to prior surgery) |
|
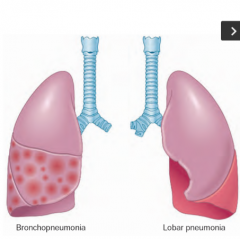
What does this show? |
Patterns of anatomic distribution for Community Acquired Bacterial pneumonia= lobar bronchopneumonia & Lobar pneumonia. Clinically differentiating is less important than identifying the infective agent |
|
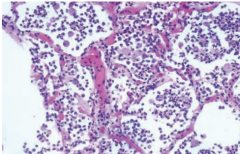
Bronchopneumonia |
Patchy consolidation of the lung that produces an exudates that is neutrophil rich and fills the bronchi, bronchioles and adjacent alveolar spaces (look at neutrophil in spaces). Creates pus forming inflammation. Most common=S. Pneumonia, S. aureus, H influenza, Klebsiella |
|
|
Stages of Lobar pneumonia |
1. Congestion (lung heavy, engorged, red). 2. Red hepatization (exudation in alveolar spaces of neutrophil, RBCs, & fibrin). 3. Gray hepatization (continued fibrinous exudates, color is gray-brown) 4. Resolution (exudates in alveolar paces is broken down and ingested by macrophages with possible fibroblast proliferation. |
|
|
What are the most common causes of atypical viral pneumonia? |
Influenza A, B, RSV, and adenoviruses |
|
|
What are the most common causes of atypical bacterial pneumonia? |
Mycoplasma pneumoniae, chlamydophila pneumonia, Legionella pneumophilia |
|
|
What is the pathophys of group 3 pulmonary hypertension? |
Acute hypoxic inhibits voltage gated K-->induces Ca++ influx triggering membrane depolarization (constriction.)
Chronic hypoxic inhibits mRNA of K channels (increased in CA+) that causes cell proliferation and inhibits apoptosis.
Result is INTIMAL FIBROSIS |
|
|
Perfusion Limited Gas Exchange |
Gas exchanged across alveolar capillary barrier is limited by blood flow in the pulmonary capillaries-->partial pressure gradient decreases along length and to increase diffusion, perfusion must be increased.
Ex: N2O and Oxygen |
|
|
Diffusion limited examples |
Carbon monoxide & oxygen with fibrosis of alveolar wall |
|
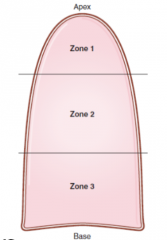
List V/Q ratios within the zones of the lung |
Zone 1: High V/Q (ventilation great, perfusion low). Zone 2: V/Q normal Zone 3: V/Q low (ventilation decreased, and perfusion increased due to gravity) |
|
|
What is a coping mechanism of the lung to remain as efficient as possible when there is a ventilation defect? |
O2 dependent vasoconstriction |
|
|
What is normal pulmonary perfusion (flow)? |
5 L/min |
|
|
What is occurring when V/Q = infinity |
A dead space is created in the lung as a region that is not perfused--no gas exchange occurs |
|
|
V/Q=0 |
A shunt is observed as blood flows through a region that is not ventilated. Can be due to an airway obstruction or right to left cardiac shunt. Pulmonary capillary blood has the same composition as mixed venous blood. Giving O2 here will have little effect |
|
|
In which of the following pathological states would the oxygen content of the trachea resemble the oxygen content into he affected alveoli? Emphysema, pulmonary fibrosis, pulmonary embolism, foreign body obstruction distal to the trachea, exercise` |
Pulmonary embolism |
|
|
What criteria is used to evaluate potentially inappropriate medications for use in older adults? |
Beers Criteria. |
|
|
Nitrofurantoin |
Prodrug, reduced by bacterial flavoproteins, inhibits ribosomal proteins (decreases protein synthesis, aerobic energy metabolism, DNA/RNA/cell wall synthesis) Bactericidal in urine=low resistance |
|
|
Tolterodine |
Anticholinergic (competitive antagonist of Muscarinic receptors). TX of overactive bladder |
|
|
What does the thyroid play a role in? |
4 B's Brain maturation Bone Growth (works with GH) Increases Beta adrenergic effects Increase basal metabolic rate |
|
|
20 year old female arrives in your clinic with complaints of neck swelling and weight gain. What is the best initial diagnostic test to order? |
TSH levels |
|
|
Levothyroxine |
Free T4 |
|
|
How does thyroid hormone travel in the blood? |
Bound to thyroxine-binding globulin (or transthyretin) *or pre albumin |
|
|
What enzyme converts T4 to T3? |
Deiodinase |
|
|
How does thyroid hormone work? |
1. T4-->T3 via deiodinase 2. T3 binds thyroid hormone receptor 3. Homodimerizes or heterodimerizes with retinoic acid receptor (most effective) 4. Removes repression hormone response element 5. Facilitates RNA polymerase activations (mRNA production coding for enzymes) |
|
|
Cretinism |
Congenital untreated hypothyroidism. Due to maternal hypothyroidism, leads to nor formed or poorly formed thyroid, iodine deficiency.
Pot bellied, Puffy-faced, Pale, Protruding umbilicus, Proterbant tongue, Poor Brain development |
|
|
Why is pregnancy associated with hypothyroidism? |
Increased thryoxine-binding globulin production (its not the amount of total thyroid hormone that determines biological activity, but the amount of Free T3 and T4). Thyroxine binding globulin bind T3 and T4, therefore it is not free |
|
|
Myxedema |
When hypothyroidism goes untreated/undiagnosed.
Glycosaminoglycans accumulated in the skin/soft tissues--> deeper voice,m acroglossia, non-pitting edema (periorbital edema, peripheral edema). Can lead to Myxedema coma: altered mental status, hypothermia, bradycardia, hypercarbia, hyponatremia. Low CO, cardiac contractility, reduced cerebral blood flow, decreased renal function |
|
|
What lab value has the highest sensitivity for primary hypothyroidism? |
Serum TSH. (if increased, then measure free T4 or free throxine index) |
|
|
Explain T3 resin uptake (T3RU) |
Tells you what % of thyroid binding globulin is unbound. Must be looked at with Free T4. If increase in T4, but decrease in T3RU, it's NOT a thyroid problem, its a increase in TBG (Prego & increased estrogen). If T4 is decreased, but T3RU is increased, you know it is a thyroid function problem and should investigate those` |
|
|
What is a bifasicular block? |
RBBB + LAFB
Most commonly caused by Epstein anomaly and ASD |
|
|
Sick Sinus Syndrome |
SA node remodeling from A fib. SA node is wonky, |
|
|
What are the indications for Statins? |
1. Known CVD 2. Ppl 40-75 w/: -DM2 -LDL70-189 3. LDL >190 4. 10 year estimated CVD risk >7.5
|
|
|
Pathophys of a fib |
-Abnormal impulses from pulmonary veins trigger initial AF. Dilation of atria and fibrosis/inflammation causes different refractory periods. Electrical reentry (impulse fails to die out after normal heart activation) leads to re-excitation. Abnormal tissues allows maintenance of AF |
|
|
Classes of A Fib |
-Acute -Paroxysmal-terminates spontaneously w/in 7 days. DC needed to stop it -Longstanding-1 year or longer, rhythm control needed. -Permanent -can't be terminated
|
|
|
Risk Factors for A fib |
-Old Age -CAD -HTN -CHF -valvular disease -pericardial/pleural diseases -diabetes -hyperthyroidism |
|
|
What procedure is necessary in A fib patients to look for thrombus before onset of cardioversion? |
TEE |
|
|
How do you treat a hemodynamically unstable A fib patient? |
Direct current cardioversion + rhythm control |
|
|
How do you treat a hemodynamically stable patient with a fib? |
Rate control` |
|
|
How do you treat an a fib patient with left atrial thrombus? |
-Rate control (beta blockers/ca+ blockers) -Anticoagulation -Cardioversion after 3-4 weeks of anticoag
-if w/ CHF use rate control w/ digoxin instead of Ca+/Beta blockers |
|
|
When is CHADS2 score applicable? |
When a patient has AFIB and you need to know if they need anticoagulation |
|
|
What are permanent pacemakers used for? |
Maintain rhythm and used to prevent bradyarrythmias
-however they don't work on preventing tachyarrthymias; ppl with pacemakers still on Bblockers and ca blockers |
|
|
What are the indications for permanent pacemaker placement? |
-SA node dysfunction (sinus bradycardia w/ sx) -Acquired AV block (increased PR interval) -Post MI (3rd degree block, persistent 2md degree AV block w/ bilateral BBB) -Neurocardiogenic syncope (carotid sinus hypersensitivity --syncope +3 sec of asystole after sinus massage) |
|
|
What are the complications of a permanent pacemaker? |
Short term: Venous access (pneumothorax, hemothroax, air embolism).
Long term: Pacemaker syndrome (fatigue, dizziness, hypotension -due to pacing ventricle asynchronously--AV dissociation). Infection, Thrombosis/phlebitis |
|
|
Where do parasympathetic innervate the heart? |
SA and AV nodes mainly (some atrial and ventricular contractile myocytes) |
|
|
Where do sympathetic innervate the heart? What do they do? |
Innervates all parts of heart (mainly ventricular muscles.
Increases rate of SA node discharge, rate of conduction, contraction force.
Releases NE which increases the permeability of myocyte membranes to Na/Ca+
|
|
|
Sinus Block |
Temporarily fails to pace for at least one cycle=NO P WAVE (or qrs). Resumes pacing in step with previous rhythm |
|
|
Primary Heart Block (1st degree) |
PR interval is >.2s (lengthens delay between A and V depolarization
-Not actually blockage of conduction.
-lengthening is consistent from cycle to cycle -Normal P-QRS-T rate -Seen in vagal states (sleep) and with drugs that affect AV conduction -Typically treatment is not indicated |
|
|
Secondary Heart Block |
allow some depolarization to conduct but block others (p dissociated from QRS) |
|
|
Wenckebach |
Secondary heart block in which a series of cycles occur with progressive blocking of AV NODE until final p way is totally blocked & associated QRS is eliminated.
Asymptomatic--no treatment |
|
|
Moritz (II) |
2ndary heart block caused by BLOCK OF PURKINJE FIBER BUNDLES that produces one normal cycle preceded by series of paced P waives that fail to conduct to ventricles (no QRS response).
Each has a consistent P:QRS ratio 3:1 or 4:1 Often has normal PR (can have prolonged but doesn't get progressively longer like Wenckebach)
Are candidates for pacemakers because it can progress to complete heart block |
|
|
What does the PR interval and QRS look like in Wenckebach? |
PR interval is lengthened QRS is normal (originates at the AV node) |
|
|
What does the PR interval and QRS look like in Moritz 2? |
PR interval is normal QRS is widened (originates below the AV node).
Problem with the PURKINJE fiber bundles being blocked |
|
|
Tertiary Heart Block |
Complete. Atria are independently paced by SA node; which the ventricles are paced at their own independent ventricular rate.
Atrial rate ~100; ventricular rate ~40
Typically see P wave superimposed with T wave
Tx: pacemaker
|
|
|
When should you suspect Hemi blocks? |
Suspect with abnormal axis shift when associated with cardiac event.
RAD--posterior hemiblock LAD--anterior hemiblock |
|
|
What supplies the SA node, AV node, bundles of HIS and posterior division of the left bundle branch with blood? |
RCA |
|
|
What supplies the RBB and anterior and posterior divisions of LBB with blood? |
LCA |
|
|
What does occlusion of LAD cause? |
RBBB with anterior hemiblock (anterior division of left bundle branch)--pg 296 in dubin |
|
|
Anterior Hemiblock (Left anterior fascicular block LAFB) |
-Block of the anterior half of the left bundle branch due to occlusion of the LCA -Shows LAD -Normal/slightly wide QRS
If person with acute anterior infarction (occlusion of LAD) has associated LAD, suspect anterior hemiblock (LAFB) |
|
|
Posterior Hemiblock |
-RAD (associated with MI or HD) -normal or slightly wide QRS -Inferior infarction (RCA) may impair blood flow to posterior division of left bundle. |
|
|
When a RBBB appears, where would you expect to see R & R prime |
V1 & V2 |
|
|
When does sick sinus syndrome most commonly appear? |
In elderly with heart disease or conditioned marathon runners (have parasympathetic hyperactivity at rest-->brady/tachycardia syndrome)
Results in no parasympatheicinnervation below AV node--> sinus bradycardia without normal escape of atrial and functional foci |
|
|
What is the etiology of sick sinus syndrome? |
Degerative fibrosis of SA node (age/idiopathic) ion channel dysfunction (inherited), and remodeling of SA node (HF, a fib, and CT diseases), OSA |
|
|
How does sick sinus syndrome look on EKG? |
Arrhythmia and presence of sx (end organ hypoperfusion + bradycardia w/ or w/o tachycardia.
Bradycardia is required for DX |
|
|
Atrial Flutter |
Sawtooth pattern. AV node blocks some of condition, determines actual ventricular rate. Flutter waves are negative in II, II, and aVF (arises just a bout tricuspid valve)
-F waves almost always at 300 bpm |
|
|
When is functional rhythm most commonly seen? |
In drug toxicity affecting AV conduction (Digoxin toxicity).
Escape when SA node turned off |
|
|
Supraventricular tachycardia |
Narrow complex tachycardia that originates about the Bundle of HIs.
QRS < .12s |
|
|
Multifocal atrial tachycardia |
Irregularly, irregular with multiple P waves (their morphologies are different. |
|
|
Acute Rheumatic Fever |
Systemic complication of pharyngitis due to group A B-hemolytic strep.
-don't worry about nephrogenic strains because they lack the M protein, therefore can't/wont lead to RF |
|
|
What type of hypersensitivity reaction is rheumatic fever? |
Type II hypersensitivity--> molecular mimicry.
Bacterial M protein antigen resembles cardiac myocytes. It shares epitopes with myosin and results in RF |
|
|
How do you diagnose rheumatic fever? |
JONES Criteria + prior evidence of Group A strep (ASO titer)
Joint-migratory polyarthritis of large joints O-heart--pancardititis, endocarditis, MITRAL VALVE REGURG, myocarditis=ASCHOFF BODIES (chronic inflammation with giant cells and fibrinoid material and anitschkow cells=histiocytes with slender wavy nuclei) N=nodules (sub Q) E=erythema marginatum S=syndenham's chorea |
|
|
What valve is most commonly affect in RF? |
Mitral Valve ALWAYS
Aortic valve may or may not be involved. |
|
|
Class I antiarrhythmic overview |
Fast Na+ channel blocker=RYTHM CONTROL -decrease the slope of phase 0 depolarization and increase the threshold for firing in abnormal pacemaker cells --only work in tachycardic state -3 groups based on effect on the length of AP
|
|
|
Class 1 Antiarrhythmics group IA |
1A= Quinidine, procainamide, disopyramide (intermediate ion channel dissociation). Use=atrial and ventricular arrhythmias (reentrant and ectopic SVT and VT). Prevents paroxysmal recurrent A fib. WPW.
MOA: increases AP duration, effected refractory period and QT interval. |
|
|
Class 1 antiarrhythmics group IB |
Lidocaine, Mexiletine, Phenytoin Fast ion channel dissociation Use: atrial ventricular arrhythmias (POST MI) digitalis induce arrhythmias. MOA: decreases AP duration |
|
|
Class II antiarrhythmic overview |
-Anti sympathetic drugs (Beta blockers)= RATE CONTROL -MOA: decreases SA and AV nodal activity by decreasing cAMP and Ca+ currents. -decreases slope of PHASE 4 abnormal pacemakers (AV node esp sensitive, increases PR interval) -Tox: impotence, exacerbation of COPD/asthma, bradycardia, CONTRAINDICATED IN COCAINE USERS |
|
|
Class III anti-arrhythmias Overview |
K+ channel blockers (amiodarone) -RHYTHM CONTROL IN AFIB/Flutter -Increase AP duration, ERP and QT interval, prolonging repolarization (K channel) |
|
|
Toxicity of Class III antiarrythmics |
Amiodarone is Class III Pulmonary fibrosis, hepatoxoicicy, hypo/hyperthyroidism, corneal deposits, blue/grey skin |
|
|
Class IV Anti-arrhythmics overview |
Slow CA+ Channel blockers: Verapamil, diltizaem). -MOA: decrease conduction velocity through AV node ==> lengthen phase 0 (upstroke) of SA node
Tox: constipation, flushing, edema, |
|
|
When would magnesium use be appropriate for a heart condition? |
When patient has tornadoes or digoxin toxicity (decreases Ca influx) |
|
|
Digoxin |
Increases contractility and simulates vagal nerve (AV node suppression--> decreased HR)
-Use: CHF, rate control in A fib too |
|
|
How do you treat triple negative breast cancer? |
Doxorubicin + radiation
Leads to increased risk of cardiotoxocity |
|
|
What is a pulse deficit? |
Difference between pulse and heart rate (pulse is lower). Suggestive of systolic dysfunction--> low EF -heart strong enough to close mitral valve ("beat") but not strong enough to open aortic valve (can't push blood out).
|
|
|
ACEi |
cardioprotective because ACE causes cardiac remodeling |
|
|
What is a summation gallop? |
S3 and S4 superimposed and heard as a single sound when HR >120 |
|
|
What are the etiologys of dilated cardiomyopathy? |
-Idiopathic (most common) -genetic (autosomal dominant mutations in TTN gene= titan) -myocarditis*** most common known cause--cox B virus) -Alcohol-direct toxic effect or due tot hiamine deficiency -Drugs-Doxorubicin, 5FU, Cyclophosphamide -After prego -glue sniffers, acromegaly -Rheumatoid arthritits |
|
|
Causes of dilated cardiomyopathy mnemonic |
ABCCCD Alcohol wet Beri Beri Coxsackie B (myocarditis) Chronic Cocaine, Chagas disease Doxorubicin toxicitiy |
|
|
What type of hypertrophy is seen in dilated cardiomyopathy? |
Essentric hypertrophy=sarcomeres added in series
Dilated cardiomyopathy causes biventricular CHF. Progressive dilation causes mitral and tricuspid regurgitation and further decreases CO. |
|
|
Pathophys of Dilated cardiomyopathy |
. LV failure--> increased EDV and ESV-->enlargement of other chambers --Progressive dilation causes mitral and tricuspid regurgitation--> Further reduced CO --Increase HR and SV and peripheral vascular resistance to compensate -Activation of RAAS bc of failing CO--> actually worsens the disease |
|
|
How is dilated cardiomyopathy diagnosed? |
No single test. Clinical picture. CXR (balloon appearance), ECHO
ECHO shows dilation with no hypertrophy, segmental wall motion abnormality, global heart enlargement and low EF |
|
|
How do you treat dilated cardiomyopathy? |
-Na+ restriction -ACEi -BBlocker -Diuretics -Digoxin -ICD -Heart transplant |
|
|
What is the most common cause of sudden death in young athletes? |
Hypertrophic cardiomyopathy |
|
|
What is the most common form of hypertrophic cardiomyopathy? |
Familiar form--autosomal dominant with complete penetrance, but varied phenotypes
Typically B-myosin heavy chain mutation |
|
|
What problems associated with hypertrophic cardiomyopathy could lead to arrhythmias? |
myofibrillar disarray and fibrosis which is present in the conduction system |
|
|
When is LVOB common? |
In left ventricular hypertrophy w |
|
|
Where does hypertrophic cardiomyopathy most commonly occur? |
IV septum of the free LV wall
Sarcomeres added in parallel (concentric) |
|
|
A patient with known hypertrophic cardiomyopathy presents to your clinic. You want to hear their murmur. What type is it most likely and what maneuvers could you have them perform? |
-Harsh systolic ejection murmur best hears along left sternal border
To increase intensity (worsen their obstruction) you need to decrease their preload. = standing, valsalva, use of inotropic drugs (digitalis)--> these changes are opposite of typical aortic stenoiss |
|
|
Pts with hypertrophic cardiomyopathy are at increased risk for sudden death. Why? |
Ventricular tachycardia or V five (correlates with LV wall thickness) |
|
|
How do you treat hypertrophic cardiomyopathy? |
BBlockers (decrease contractility to decrease myocardial workload), decrease heart rate, non-dihydropyridine CA channel blocker (verapamil==second line), implantable cardiovertor defibrillator).
Screen all first degree relatives with echo
Avoid=digoxin, ARBs, diuretics |
|
|
Causes of restrictive cardiomyopathy |
Amyloidosis, myocardial fibrosis after surgery, radiation, hemochromatosis, Loffler syndrome (endomyocardial fibrosis with eosinophilic infiltrate), endocardial fibroelastosis as kid, sarcoid,
Decreases ventricular compliance, diastolic HF |
|
|
Restrictive Cardiomyopathy is an example of what type of heart failure? |
diastolic |
|
|
Pathophys if doxorubicin toxicity |
Induction of toxic O2 free radicals--> oxidative stress--> lipid peroxidation of membranes -->vacuolation-->irreversible damage and myocyte replacement by fibrous tissue
--increase ROS-->increase Ca+ relase --Mitochondrial damage--doxo binds to cardiolipin-->disrupts ETC-->decrease ATP --Forms complexes with Fe--> lipid peroxidation and free radical generation |
|
|
What med can you do to prevent doxorubicin cardiomyopathy? |
Dexrazoxane
It's an iron chelator that reduces ROS formation |
|
|
What is the most common cause of ischemia leading to systolic dysfunction of the heart? |
CAD |
|
|
Paroxysmal nocturnal dyspnea |
Breathless awakening from sleep.
Without effects of gravity, fluid from intestinal spaces moves into vascular compartment--> increases venous return from redistribution of blood---> backs up in lungs
Sign of heart failure |
|
|
Pulmonary edema pathophys |
Hydrostatic Pressure >oncotic Pressure
Transudation of fluid and accumulation of hemosiderin laden macrophages in lung |
|
|
Treatment of systolic heart failure |
-Lifestyle modification=stop smoking, alcohol, salt/fluid, weight loss -Treats sx: diuretics -modify disease: ACEi (first line) and B-blockers( slows HR, allows more filling=results in increased EF)** first line combo*** |
|
|
What heart drugs are best utilized for African American patents? |
Hydralazine and Isosorbide dinitrate |
|
|
What are the key clinical sx of diastolic failure? |
HTN Rales S4 LVH |
|
|
ACCF/AHA stages of HF |
-Stage is determined upon diagnosis and CANNOT be changed even though patients sx can improve
A: High Risk/lots risk factors, no heart disease sx B: Structural heart disease without signs or sx C: Structural heart disease w/ prior or current sx D: Refractory HF requiring specialized interventions |
|
|
How do you treat stage A HF? |
-Heart healthy lifestyle -prevent CAD/LV structural abnormalities -Control HTN/Lipid disorders/obesity/smoking/DM |
|
|
How do you treat stage B HF? |
-Prevent HF sx & cardiac remodeling -ACEi & -B-blockers (in all pts with reduced EF) -Statins -those who need valve replacements/ICD
***Don't give nohydropyridine Ca channel blockers because harmful!! --amlodipine is exeption |
|
|
How do you treat stage C HF? |
Goals: control sx, improve quality of life, prevent hospitalization/mortality/identify comorbidities, tx, educate on lifestyle
1st line=ACEi (ARB) +Bblocker -if volume overload +loop -if EF<35% or post MI EF<40% and creatinine >30, and K <5, + spiroclactone -digoxin (dec hospitalization, not mortality) -anticoag if post stroke or afib -statins (not beneficial), fish oil (is beneficial) |
|
|
How do you treat class D HF? |
Same as stage C
1st line=ACEi (ARB) +Bblocker -if volume overload +loop -if EF<35% or post MI EF<40% and creatinine >30, and K <5, + spiroclactone -digoxin (dec hospitalization, not mortality) -anticoag if post stroke or afib -statins (not beneficial), fish oil (is beneficial)
-fluid restriction (esp if hyponatremia) -IV inotropic support (pts with cardiogenic shock) -mechanical circulatory support -cardiac transplant |
|
|
NYHA Functional Classification of HF symptoms.
Class I |
No limitation of physical activity, ordinary activity does not cause HF sx. |
|
|
NYHA functional classification of HF sx.
Class II |
Slight limitation of physical activity, comfortable at rest, but ordinary activity causes sx |
|
|
NYHA functional classification of HF sx.
Class III |
Marked limitation of physical activity, comfortable at rest, but ordinary physical activities results in sx
Aka: sx with restricted activities |
|
|
NYHA functional classification of HF Sx
Class IV |
Unable to carry on physical activity w/o sx of HF at rest
Sx at rest |
|
|
When is a cardiac transplant contraindicated? |
--Irreversible pulmonary HTN (unless get heart and lungs together) --Cancer --Infection |
|
|
Indications for Implantable cardioverter defibrillator (ICD) |
-Tried medical treatment for 3 months with EF<35% (30% sometimes) -hx of MI/CAD and EF <35% |
|
|
Define Triple Negative Breast Cancer |
-Absence of estrogen, progesterone and EGFR2 (HER2) markers
-bc of this, can't treat with anti-HER2 or hormones
-Very sensitive to chemo though--taxanes (Paclitaxel) and anthracyclins (doxorubicin) |
|
|
MOA of doxorubicin |
Intercalate into DNA and blocks DNA/RNA synthesis and interferes with Topoisomerase II
Binds to cell membrane-->alters transport process
Generation of O2 radicals-->cardiotoxicity (bc heart has little SOD to protect it) |
|
|
MOA of Paclitaxel |
Microtubule stabilizes (G2/M)-->inability of cell to divide by mitosis=death |
|
|
What is the pathophys behind S4? |
Atria contracting against a stiff ventricle--> scar tissue or hypertrophied ventricle (high atrial pressure)
Diastolic dysfunction |
|
|
Pulse deficit |
Occurs when heart beats (systole) but the contraction is not sufficient to transmit pule to periphery--> heart rate is faster in auscultation than in periphery |
|
|
What is the standard of care for intestinal lung disease form nitrofurantoin? |
1. Discontinue Nitro 2. Begin corticosteroids 3. Oxygen supplementation if needed |
|
|
How do you dx chronic form of lung disease associated with nitrofurantoin? Acute? |
Open lung biopsy=Chronic
You can dx acute reaction to nitro solely by the characteric symptoms and the duration of time between drug exposure and symptom onset |
|
|
How does restrictive lung disease effect FEV1 and FVC? |
In restrictive lung disease, usually there is symmetric or near symmetric decrees in FEV1 and FVC. That leads to normal FEV1/FVC ration
Obstructive causes a bigger decrease in FEV1 than FVC=lowe FEV1/FVC ratio (less than 80% predicted) |
|
|
What is the pathophys of acute nitrofurantoin lung injury? |
Acute=hypersensitivity (type I or III)
Chronic= allergic or toxic response |
|
|
What does the histopathology of acute and sub acute nitrofurantoin pulmonary injury look like? |
-Vasculitis -Mild interstitial inflammation -eosinophilia, -Reactive type II pneumocytes -Focal hemorrhage -small organizing microthrombi (rare)
Chronic disease=diffuse interstitial fibrosis |
|
|
What does chronic nitrofurantoin lung injury look like on histology? |
Diffuse interstitial fibrosis |
|
|
What are risk factors for statin induced myopathy? |
-Females -Advancing age -Lower BMI -DM -HTN -other meds that interact with CYP3A4 -untreated hypothyroid -renal & hepatic disease -SNP on SCLO1B1 gene of Chr 12 |
|
|
Proposed mechanisms of myotoxicity by statins? |
1. Reduced sarcolemmal cholesterol--> membrane destabilization
2. Isoprenoid depletion--> isoprenoids are lipids and linked to controlling apoptosis (if gone-->induced apoptosis)
3. Inhibition of CoQ10--> isoprenoid in ETC-->impaired mitochondrial respiratory function-->myopathy |
|
|
Which statins are most likely to induce myopathy? |
Dose dependent=more drug=more toxicity
Atovastatin Simvastatin (80mg highest dose=most myopathies) |
|
|
Cushing Reaction |
1. Bradycardia 2. HTN 3. Respiratory depression
--increased intracranial pressure contracts arterioles--> cerebral ischemia and reflex sympathetic increase in perfusion pressure (hypertension)--> increase stretch--> reflex baroreceptor induced bradycardia. |
|
|
FAST scan |
Looking for blood/fluid in -Perihepatic space -perisplenic space -Pericardium -pelvis
-DOESN'T LOOK AT LUNGS FOR PNEUMO/HEMOTHROAX, unless is says eFAST (for extended) |
|
|
If a patient is on a ventilator and you want to increase their O2 concentration, how would you do it? |
Increase FIO2
or
Increase PEEP |
|
|
If a patient is on a ventilator and you want to decrease their CO2, how would you do it? |
Increase Respiratory Rate
or
Increase Tidal Volume |
|
|
What type of ventilator support puts the patient at greatest risk of blowing out a lung? |
Volume-Limited ventilation
-The volume the ventilator is set at could exceed the volume capacity of the pts lungs.
Pressure limited ventilation reduces this risk |
|
|
When do you start nutrition for critically ill patients? |
Early (within 48 hers)--enteral nutrition (directly to GI tract)--Decreases Mortality!
If pt has contraindications to internal nutrition, do not start parenteral (IV) feeding before 1-2 weeks bc of risk of infection |
|
|
How do you interpret ABG? |
1. pH 2. Determine cause --low HCO3 or High PCO2 3. Calculate anion gap (correct for albumin +2.5 for every 1 drop) 4. (Anion Gap-12) +HCO3=24 -if =24, perfect metabolic acidosis - if >24, metabolic alkalosis - if <24, extra uncounted anion 5. Compensation (via ventilation) -1.5 (HCO3) +8 (+/-2)=expected PCO2 -if more--resp alkalosis on tom of metabolic - if less, respacidosis -->hyperventilate pt |
|
|
What is the general rule for detecting an MI when given both CK and CKMB? |
If CKMB is less than 4% of CK, then there is NOT AN MI |
|
|
Pigment associated renal failure |
--Acute kidney injury due to non protein heme pigment released from myoglobin--->toxic
Rhabdo |
|
|
MOA of enoxaparin
When would you not want to use it? |
Specific inactivation of Xa
--When need to reverse its effects: Prego pts and surgery pts |
|
|
General rule to determine if LB is traumatic? |
For every 750 (saw ranges from 500-1500) RBC, allow 1 WBC |
|
|
What are indications for tube feeding? |
-Functional GI tract but cant/wont ingest orally -prolonged anorexia -Severe protein-energy undernutrition -Coma -Liver failure -Head/neck trauma -critical illness causing metabolic stress |
|
|
What are the most common ventilator associated pneumonias? |
1. Klebsiella (G- Rod) 2. Pseudomonas (G-Rod) 3. S. Aureus (G+ cocci) |
|
|
What is the pathophys behind t(15;17)? |
Retinoic acid receptor (17) translocates with a PML novel gene on chr 15, resulting in overproduction of promyelocytes
PML-RAR fusion blocks transcription via deacetylation of Histone--> preventing maturation of promyelocytes--->accumulation of them and precursors=blasts |
|
|
What are risk factors for acquiring APL? |
-Past Topoisomerase II inhibitor use (etoposide, anthracycline (danorubicin) -Having cancer (APL can be a secondary cancer) |
|
|
What markers does APL present? |
CD34+ HLA-DR- DC56+ MPO+(nonspecific for AML)
-t(15;17) |
|
|
How does the hyperglandular type of APL present? |
-With LOW WBCs!-->pancytopenia -excess promyelocytes -less blasts, but hypergranulated -AUER rods --faggot cells (bundles of AUER rods)
APL in general--abrupt, young adults (30-40), thrombocytopenia |
|
|
How do you treat APL? |
Induction therapy: ATRA (all trans retinoic acid) & Daunorubicin (anthracycline based chemo)
Consolidation therapy--> ATRA + Daunorubicin + Cytarabine
-in general, don't forget about rasburicase/allopurinol to prevent tumor lysis syndrome |
|
|
What type of AML would most likely cause a patient to present with increased PT/PTT, decreased fibrinogen, thrombocytopenia and positive D-dimer? |
APL (Acute promyelocytic leukemia)
-has the highest risk for DIC (described) -due to either hypercoagulability of auer rods or from promyelocytes releasing procoagulant substances |
|
|
What is maintenance therapy for APL? |
ATRA + antimetabolite (methotrexate +mercaptopurine) for 1-2 years |
|
|
What is an indicator of likely relapse in an APL patient?
How do you treat relapse? |
WBCs above >10,000 at initial presentation (APL has uncommonly low WBCs)
-Arsenic trioxide (ATO) -interacts with PML-RARa protein-- -Methotrexate -Cytarabine (if CNS involvement) |
|
|
Where does relapse of APL commonly occur? |
CNS (in our pt, leukemic meningitis) & skin manifestations
|
|
|
What is the prognosis for APL? |
70-80% cure rate (unlike most leukemias)
-extramedullary relapse (CNS or SKIN) is associated with a poor prognosis |
|
|
What marker are all AMLs positive for?
|
MPO +
MPO can crystallize into an auer rod |
|
|
Which leukemia are down syndrome patients most likely to get before the age of 5? |
AML |
|
|
Which type of leukemia are down syndrome patients most likely to get after the age of 5? |
ALL
ALL--> presents most commonly in kids |
|
|
What puts a patient at increased risk to get AML? |
-Previous cancers treated with alkylating agents or radiotherapy -development from myelodysplastic syndromes (hypercellular bone marrow, but not blasts >20%) |
|
|
What marker is positive in all ALLs? |
Tdt+
-DNA polymerase only present in nucleus of lymphoblasts |
|
|
What markers are positive in B-ALL? |
CD10 CD19 CD20 CALLA PAX5
-If t(9;22) is present in B-ALL=poor prognosis |
|
|
Which cancer requires prophylaxis to scrotum and CSF directly? |
B-ALL |
|
|
What markers are positive for T-ALL? |
CD2 -8
Doesn't express CD10 |
|
|
What is the most common presentation of T-ALL? |
Anterior mediastinal mass in a teenage boy
T-cell lymphoma! |
|
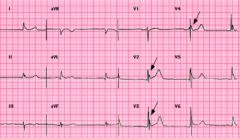
What could cause this? |
Osborn Wave (most prominent V2-V5
-Hypothermia -subarachnoid hemorrhage -brain injury |
|
|
What cardiac changes on an EKG can hypothermia cause? |
-Prolonged intervals (slowed K channels) -Osborn waves -shivering can cause baseline abnormalities (not a fib) -Arrhythmias |
|
|
Sarcolemma injury causing release of myoglobin, CK, uric acid, electrolytes from myocytes into circulation |
Rhabdomyolysis |
|
|
Generally, what is DIC? |
Pathologic activation of fibrin thrombi in the microvasculature causing the consumption of platelets, fibrin, and coat factors -->secondary activation of fibrinolysis |
|
|
How does AML and adenocarcinoma increase the risk for DIC? |
-Mucins & immature cells from these cancers directly activate factor X in the clotting cascade
-circulating tumor cells also express TF and cancer procoag (also activates X) |
|
|
What cytokine is responsible for inducing endothelial cells to express TF on surfaces and decrease the expression of thrombomodulin in the setting of DIC? |
TNF
--it most commonly does this in the setting of septic shock because TNF is released from lipoteichoic acid in G+ bacteria |
|
|
Complications of DIC? |
-superficial thrombophlebitis (trousseau)-venous -arterial thrombosis -inschemi renal infarct, stroke -PE -Bleeding diathesis |
|
|
What is the best screening test for DIC? - |
d-dimer |
|
|
Pathogen associated with HUS? |
-E.coli 0157H7 (Shiga toxins) or shigella
Hemolytic aniema, peripheral thrombocytopenia, and renal failure |
|
|
How do you treat nosocomial pneumonia if the patient has risk factors for multidrug resistant pathogens? |
-Anti-pseudomonas (pipercillin-tazobactam/imipenme) -AND anti-pseudomonas fluoroquinology (cipro) -AND linezolid, vanc, or televacin |
|
|
What are the complications of HCT? |
Short term=mouth sores, D/N/V, hair loss, Lung, bone, liver issues GVHD
Long term-Fertility probs, additional cancers, long lasting GVHD |
|
|
Hyperacute rejection |
Minutes
Pre-existing recipient antibodies react to donor antigen
Hypersensitivity type II--> activates complement--> thrombosis of graft--> ischemia (remove graft) |
|
|
Acute Rejection |
Weeks/months
-Cellular (CTLS against donor MHC) -humoral-antibodies in recipient develop after transplant -vasculitis of graft vessels w/lymphocytic infiltrate -Can prevent/reverse with immunosuppressants |
|
|
Chronic rejection |
-MHC of donor is perceived by CTLS as self presenting a non-self antigen--> causes irreversible T cell and antibody mediated vascular damage--> fibrosis of graft tissue and blood vessels |
|
|
GVHD |
Grafted immunocompetent T cells proliferate in immunocompromised host and reject host cells with foreign proteins
--This is helpful in HCT because helps eradicate the not killed yet cancer cells! |
|
|
If a patient presents with a solitary fibrinogen deficiency, what would you administer to correct it? |
Cryoprecipitate |
|
|
Stenotrophomonas Maltophila |
G- Rod--causes pneumonia in vented pts -Transmitted via medical devices and healthcare workers -Tx: TMP-SMX Virulence-extremely antibiotic and disinfectant resistant |
|
|
Acinetobactor spp |
G- Rod--- causes pneumonia, line related bacteremia, burn wound infections, eye infections --transmitted via medical devices and hands of healthcare workers -Virulence: multiple acquired mechs of resistance. ANTIPHAGOCYTIC CAPSULE
|
|
|
How long can a patient be on translaryngeal incubation before switching to a trach tube? |
-ENT guidelines suggest 3 days -ABSOLUTE longest is 3 weeks |
|
|
Hypoxemic respiratory failure |
-Causes: shunt, V/Q mismatch, hypoventilation, low SvO2, diffusion impairment -Hypoventilation is the only cause with a normal A-a gradient -Low SvO2 can be due to anemia, hypoxemia, increase O2 consumption or low cardio output (after cardiac surgery/cardiogenic shock) -Can present with Anxiety and agitation |
|
|
Causes and symptoms of hypercapnic respiratory failure |
1. Alveolar hypoventilation--> decreased repiratory drive (CVA, neuromuscular disease, drugs (benzo, NMB, anesthetics, narcotics ) increased mechanical load (effusion or fibrosis), obesity, excessive dead space (COPD, PE, fibrosis)-->should compensate by increasing RR, but can't
2. Increased CO2 production--> sepsis, sirs, major surgery, anxiety/pain and overfeed (tachypnea)
Sx: Somnolence. |
|
|
When should a patient be considered to be put on a ventilator? |
-RR>35/min -Respiratory acidosis with pH<7.2 -AMS -VC<15ml/kg or max inspiratory pressure <25 -PaOC<55mmHg when Fi02 1.0 |
|
|
What are causes of a respiratory shunt? |
Airway obstruction-most common
V/Q=0
|
|
|
What findings would you expect on respiratory exam of a patient with pleural effusion? |
-Decreased resonance and tactile fremitus -Decreased breath sounds |
|
|
What labs must you order if you suspect a patient has a pleural effusion? |
Serum LDH, protein
LDH, Protein, and pH of pleural fluid
--These are needed to classify effusion according to lights criteria
|
|
|
What are the most common causes of pleural effusions? |
CHF>pneumonia>malignancy>PE>viral disease |
|
|
Decreased DLCO Increased A-a gradient ~normal FEV1/FVC ratio |
Interstitial lung disease
-Shows a restrictive pattern in which FEV1/FVC are near normal |
|
|
Idiopathic pulmonary fibrosis |
-Unknown cause -POOR PROGNOSIS -6-7th decade -HRC shows UIP (fibrosis, honeycombing) |
|
|
ILD-Sarcoidosis |
-CD4 T cells involved -Noncaseating granuloma -Presents 20-50s -good prognosis (1/3 get chronic disease) -Tx: steroids AND DO EYE EXAM (for uvitis) |
|
|
Extrinsic allergic alveolitis (means in involves alveolar walls) |
Aka -Hypersensitivity pneumonitis
-RF: >65, M>F, -Tx: avoid antigen; steroids -Caused by known inhaled antigen exposure--> type III/IV HSTR -if chronic==>clubbing, chronic neutrophilia -Appears as loose granuloma with lymphocytic infiltrate |
|
|
Who is at greatest risk of hyaline membrane disease? |
Surfactant deficiency--> alveolar collapse-->PHTN -Premature babies -Babies of diabetic mothers -Those delivered via C-section
-Tx: maternal steroids before delivery and surfactant for baby |
|
|
People exposed to asbestos are at greatest risk to get what disease? |
LUNG CANCER!!!
--Although mesothelioma risk is increased as well, lung cancer risk is higher |
|
|
What diseases are associated with increased risk for ILD? |
RA, Scleroderma, SLE, poly and dermatomyositis, Sjorgren's, MCTDs, ankylosing spondylitis, psoriatic arthritis, Behcet's disease (vasculitis),
-UIP and NSIP both common on histology |
|
|
What tests would you perform if you suspect a patient has Wegener's disease? |
c-ANCA CXR
Tx: cyclophosphamide & corticosteroids |
|
|
What is the pathophys behind granuloma formation? |
Th1 cells secrete INF gamma--> activate macrophages-->machrophages secrete TNF alpha--> induce/maintain granuloma formation
Therefore treatment with anti TNF drugs work |
|
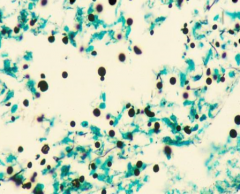
Dx? |
Cryptococcus (found in soil and pigeon poop) 3-7 to 20-25 um (LARGE VARIATION IN SIZE) -most commonly causes meningitis in immunocompromised (ring enhancing lesion in CNS) -Stain for it using India ink -Latex agglutination-->detects capsular antigen
|
|
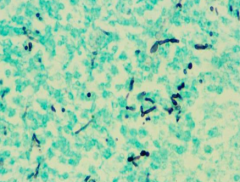
Dx? |
Candida 10-12u Pseudohyphal bud |
|
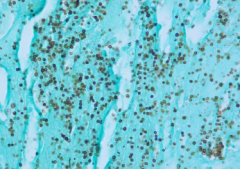
Dx? |
Histoplasmosis Grocot stain (+ means its a fungus) -Hyphal and yeast forms (this is yeast(
|
|
|
What is the syndrome called when a granuloma forms from pneumoconiosis (most commonly coal worker's lung) and has associated RA? |
Caplan Syndrome |
|
|
Caseating granuloma with prominent eosinophils in a young man who has a pmh of asthma? |
Churg-Strauss Syndrome
-Test p-ANCA |
|
|
Anthracosis |
Collections of carbon laden macrophages found in people who live in polluted areas
-no clinical significance
-bad only if it leads to coal worker's lung |
|
|
Which pneumoconiosis disease has an increased risk for TB? |
Silicosis--- is the most common occupational induced ILD (sandblasters)
-fibrosis nodules in the upper loves of the lung which impair PHAGOLYSOSOME formation |
|
|
What changes to the lung occur as we age? |
Decreased- lung elasticity, FVC, Diffusion capacity, arterial oxygen, respiratory muscle strength
Increased--FRC, RV,
TLC stays the same (unless altered by disease (emphysema))
|
|
|
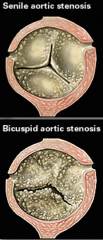
What is the most common cause of stenosis in elderly patients? |
Calcified aortic stenosis from wear and tear
-causes LV outflow obstruction during systole-->concentric LVH and post synoptic dilatation of the aorta due to the pressure of blood impacting the wall of the aorta in order to pass through. |
|
|
How can aortic stenosis lead to ischemia? |
Decreased blood is able to get through aortic valve--> this means coronary arteries get less blood during diastole-->the heart itself gets less blood---> angina/ischemia |
|
What is a complication of this condition that could be seen on labs/blood smear? |
Microangiopathic Hemolytic Anemia-->schistocytes on blood smear
--indicates the need for a valve replacement |
|
|
Medicare O2 indications |
-PaO2 <55mmhg -SaO2<88% -PaO2 between 55-60, SaO2 9% with evidence of pulmonary HTN, peripheral edema suggestion CHF or Polycythemia (HCT>55%) |
|
|
In what granulomatous disease would a patient present with anergy to a TB skin test? |
Sarcoidosis---CD4 lymphocytes are used to form granuloma and not able to respond |
|
|
What lab finding could be found in all patients with noncaseating granuloma (specifically those with sarcoidosis)? |
Hypercalcemia
Alpha 1 hydroxylase activity of the epitheliod histiocytes converts vitamin D to its active form--> absorb more calcium |
|
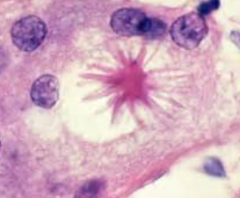
The presence of these on histology suggest what dx? |
Stellate bodies=Sarcoidosis
Funny configuration of giant cells |
|
|
Smoking related interstitial disease |
-Desquamative interstitial pneumonia--good response to steroids
-Respiratory bronchiolitis Associated interstitial pneumonia--only gets better with smoking cessation
-Both have smokers macrophages (dusty brown pigmented) |
|
|
In pulmonary hypertension, what pathologic things happen to the intima and media? |
Medial hypertrophy & Intimal fibrosis of the pulmonary arteries |
|
|
A young female presents with difficulty berthing when she carries her groceries from her car to her apartment. Her pulmonary pressure is 43mmHg. What is the most likely underlying genetic mutation? |
BMPR2 inactivating mutation
Leads to proliferation of vascular smooth muscle |
|
|
How do you treat pulmonary HTN? |
-lung transplant (but not if heart is source of it, then would need both transplanted) -treat underlying disease -Oral Ca+ channel blockers -phosphodiesterase 5 inhibitors (2nd line) -Na restricted diests -Avoid high altitude -pregnancy termination |
|
|
Destruction of elastic tissue in the lungs is best portrayed by what disease? |
Emphysema--> obstructive disease because can't get air out. Like breathing into a plastic sac and expecting it to blow the air back at you like a balloon would.
AAT Def--> not enough antiproteases Smoking-->> too many proteases |
|
|
Pt presents with cough of 6 month duration. Lung biopsy reveals hypertrophy of mucus secreting glands in the bronchioles. What is the dx, and what do you use to measure the amount/# of glands? |
Chronic Bronchitis (Blue Bloater)
The Reid Index is used to indicate gland hypertrophy (mucus gland depth/total thickness of bronchial wall) if >50%=pathologic |
|
|
In what disease would patients exhales through pursed lips to increase airway pressure and prevent collapse during respiration? |
Emphysema (pink Puffer)
-Increase elastase activity results in increased compliance of the lung and decreased recoil of lungs because of the damage done to alveolar walls |
|
|
What is permanent dilation of the airways called? How does it present?
|
Bronchiectasis -Necrotizing inflammation of the bronchi with damage to the walls -Sx: purulent sputum, recurrent infections hemoptysis
|
|
|
Who is at greatest risk for bronchiectasis? |
Permanent dilation of the airways (like me trying to blow through a pipe the size of my head and expecting to feel the air on the other side).
-CF pts -Kartageners syndrome -pts with tumors -Necrotizing infections -allergic bronchopulmonary aspergillosis |
|
|
How might a patient with known sleep apnea present with hyperviscous blood? |
Sleep apnea=>hypoxia-->increased epo release--->more RBCs (erythrocytosis)-->hyperviscous blood |
|
|
Which was does the trachea deviate in a spontaneous pneumothorax? |
Same side as the pneumothorax
Spontaneous Same Side-->all S |
|
|
Which side does the trachea deviate in a tension pneumothorax? |
away from the side of the lesion |
|
|
What is the SPHERE of complications of lung cancer? |
Superior vena cava syndrome Pancoast tumor Horner's syndrome Endocrine (paraneoplastic syndromes) Recurrent laryngeal sympts (hoarseness) Effusions (pleural or pericardial) |
|
|
Causes of transudate pleural effusions |
CHF, liver cirrhosis, nephrotic syndrome, PE, fluid overload (like IV or transfusion overload) |
|
|
Causes of exudative pleural effusions |
Malignancy pneumonia collagen vascular disease Trauma anything with increased permeability |
|
|
What is the formula for corrected reticulocyte count? |
(Patient HCT/45) X RET Count =corrected
-If polychromasia is present in smear divide by 2
If the corrected reticulocyte count is above 3 in an anemic patient, it is appropriate. If it is below, it is not. |
|
|
What lab value do you look at to differentiate anemia of chronic disease from iron deficiency anemia when they are both normocytic? |
Serum ferritin.
Iron deficiency will have low ferritin and anemia of chronic disease will have high ferritin because it is busy hiding all the iron in the liver |
|
|
What is aplastic anemia? |
-Inherited or acquired mutations in the TERT gene (causes shortened telomeres). -Causes pancytopenia with hypocellular bone marrow with fatty replacement
-Acquired causes include: antimetabolites, alkylating agents, chemical agents (benezene, insecticides (DDT)), TB, EBV< parovirus, hepatitis, ironizing radiation, THYMOMA, paroxysmal nocturnal hemoglobinuria
|
|
|
Extravascular hemolysis causes |
RBCs are either coated with IgG (Warm) or IgM (Cold) or are misshapen
-macrophages of the spleen eat them (why called extravascular-->death not in the vasculature)
-the heme and protoporphyn break down causing elevations in unconjugated bilirubin and protoporphyn |
|
|
Intravascular hemolysis causes |
-Enzyme deficiency (G6PD) -Complement distruction -Mechanical damage
Increased plasma and serum hemoglobin, increased LDH, and hemosiderinuria |
|
|
Hereditary eplitocytosis |
-AD mutation in spectrin and band 4.1 (structural proteins of RBC) -"Spectacle for one person"=spectrin 4.1 (spectacles are elliptical shaped) -Elliptocytes account for >25% of RBCs -Extravascular hemolysis |
|
|
Hereditary spherocytosis |
-AD mutation in spectrin, ankrin, band 2 and band 3--results in dehydration of the cell and spherocyte formation -Unconjucated bilirubin is extremely elevated (gallsotones) -spherocytes and ELEVATED MCHC (only anemia with this that we know of!) |
|
|
Paroxysmal nocturnal hemoglobinuria |
-Deficiency of anchors for CD59, CD55, DAF (decay accelerating factor) on platelets, RBCs, and neurtophils--->suscepitble to compliment damage! -when sleep, become slightly acidotic-->activates complement -If deficiency of those anchors-->complement destroys cells at night!
-DX: find missing anchor CD55 |
|
|
What is the mutated substitution in sickle cell? |
Valine for glutamic acid at 6th position in the beta chain |
|
|
Pathophys of sickle cell anemia |
-sickles themselves cause no problem -When cells are deoxygenated they form sickles -acidosis, volume depletion, and hypoxemia increases the change of sickling as well. -recurrent sickling causes membranes to be damaged--->marked for distruction by the spleen! |
|
|
What are the first signs of sickle cell anemia in an infant? |
Dactylitis (swelling of hand and feet)
Dx: using hemoglobin electrophroesis
|
|
|
G6PD deficiency |
X-linked recessive disorder -predominately intravascular hemolysis -reduced production of glutathione (anti-oxidant) -Excess ROS oxidize hemoglobin and form Heinz Bodies -When these RBCs go through the spleen, the splenic macrophages remove the heinze bodies-->form a BITE CELL |
|
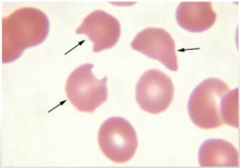
Most likely cause? |
-Removal of Heinz bodies forming BITE Cells -G6PD |
|
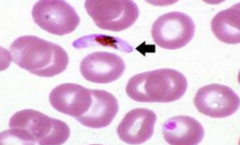
What is this? Most likely vector? |
Organism in RBC-->think Malaria
-Anopheles mosquite bite = vector Plasmodia malaria species--->sporozytes infect the liver-->then released and invated RBCs causes rupture-->intravascular hemolysis |
|
|
What does a positive direct coombs test tell you?
aka "Direct antiglobulin text" DAT
Ddx? |
There are antibodies attached to the RBCs themselves
DDX: -Transfusion reaction -Hemolytic disease of the newborn (mother's ab cross the placenta and attack fetal rbcs) -AIHA (Cold=IgM; Warm=IgG) -Drug induced (alpha methyldopa, levodopa, quinidine, high dose penicillin, 3rd gen cephlo) -hypergammacolbuinemia
|
|
|
What does Indirect Coombs test? |
If there are antibodies in the serum against RBCs (not if there are ab on the surface of RBCs like Direct)
-Used most often to check to make sure blood and patient are compatible before transfusion
|
|
|
What is the Ddx of a positive indirect coombs test? |
-Isoimmuniation from previous transfusion -Autoimmune HA -incompatible blood or medication |
|
|
What are antibodies created against in heparin induced thrombocytopenia? |
Antibody to platelet factor 4
--activates platelets-->they aggregate=decreases platelet #s, increased risk of thrombosis (MI, PE, end organ damage) |
|
|
What are the symptoms of primary bleeding disorders? |
Primary disorders-->problems with platelets mucosal bleeding (epistaxis hemoptysis, GI bleeding hematuria menorrhagia 0intracranial bleeding occurs if there is severe thrombocytopenia |
|
|
What is the most common cause of thrombocytopenia in children and adults? Explain it. |
Immune Thrombocytopenic Purpura (ITP)
-Autoantibodies from plasma cells in spleen -Splenic machrophages then eat the platelet that are bound by the antibodies -usually self limited in children after a viral infection or immunizations -Chronic forms in adults usually present in SLE pts or women of child bearing age (can transfer to baby by bc IgG can cross placenta, but is short lived)
|
|
|
How do do you treat immune thrombocytopenic purpura? |
-Initially corticosteroids -IVIG sometimes -Splenectomy if severe (gets rid of place where plasma cells make auto antibodies and where macrophages eat the platelets) |
|
|
What causes TTP? |
Decreased ADAMSTS13 (usually an acquired autoantibody in adult females)---even though our case was an adult male |
|
|
What does ADAMTS13 do? What disease is it defective in? |
Its like a lawnmower. It mows off long strands of vWF, preventing platelet aggregation from getting to big.
TTP |
|
|
What causes HUS? |
E.Coli O157:H7
The e. Coli damages endothelial cells, resulting in platelet microthrombi |
|
|
Bernard Soulier syndrome is due to what? What would you find on blood smear? |
Deficiency of GPIb (holds platelets to vWF)
Blood smear shows enlarged platelets (Big Suckers) |
|
|
Glanzmann thrombastenia is due to what? |
Genetic deficiency of GPIIb/IIIa = holds platelet to platelet |
|
|
Why would chronic kidney disease affect the adhesion and aggregation of platelets? |
CKD commonly results in uremia.
Uremia disrupts platelet function |
|
|
Signs of secondary hemostatsis dysfunction |
Deep tissue bleeding into muscles and joints Rebleeding after surgical procedures (sircumcisio and wisdom took extration |
|
|
What lab values would you expect from a patient with hemophilia A? |
Deficiency of factor VIII
-PTT increased -PT normal -normal platelet count |
|
|
Von Willebrand Disease |
AD--->decreased vWF -vWF is from Weibel palade bodies and signals platelet adhesion -vWF normally stabilized factor VIII, so labs show an increased PTT |
|
|
What test is used to dx Von Willebrand Disease? |
Ristocetin test-->induces platelet agglutination by causing vWF to bind platelet GPIb;
if patient lacks vWF--->no agglutination=abnormal! |
|
|
How do you treat Von Willebrand disease? |
Desmopression (ADH analog)
---this increases vWF release from Weibel Palade bodies |
|
|
Which coagulation factors are carboxylated by vitamin k? |
2,7,9, 10, protein C and S |
|
|
What could cause a vitamin K deficiency that would result in clotting deficiencies? |
-Newborns (due to lack of GI colonization of bacteria which synthesize vit K. -Long term antibiotic use -Malabsorption--leads to deficiency of fat-soluble vitamins (ADEK)===>worry about in CF pts |
|
|
How do you treat TTP?
How do you treat HUS? |
TTP=plasma exchange (replaces ADAMTS13, removed inhibitory antibodies)
HUS-Supportive care |
|
|
What are distinguishing symptoms that help you differentiate clinically between TTP and HUS? |
TTP=neurological abnormaltities; more common in adults
HUS==see renal failure (think of the name); more common in kids |
|
|
A 27-year-old male presents to the emergency department with a |
Deficiency in degredation of von Willebrand Factor |
|
|
What doe chylomicrons do? |
Transport DIET DERIVED triclycerides in the blood.
This means they are absent during fasting
-made of 90% TG |
|
|
What are the components of LDL? What are its defining apoproteins? |
100% cholesterol (why it is so bad!)
B100 |
|
|
Antibody mediated TRALI |
Transfuse HLA antibodies or human neutrophil antigen antibodies HLA antibodies may bind to endothelial cells and activate the neutrophils or •HNA antibodies will bind directly to the neutrophil and activate it. •HLA/HNA antibodies form due to exposure to fetal alloantigens during labor --> more deliveries, higher risk In patients receiving transfusions, they can also be sensitized! |
|
|
Non-Antibody mediated TRALI |
Non-Antibody mediated TRALI This is mostly a consequence of blood storage. –Multiple explanations have been offered, but nothing is definitive. –Bioactive lipids or accumulated soluble CD40 ligand (speculative)
|
|
|
How do you treat suspected TRAIL? |
STOP TRANSFUSTION |
|
|
Kwashiokor |
Severe protein malutrition -Results in skin lesions (flaky paint) appearance, edema, ascities, diarrhea, anemia, HSM, liver malfunction (fatty change due to decreased apolipoprotein synthesis) -Pt also seems listless, apathetic and anorexic
-Think small child with swollen belly |
|
|
Marasmus |
Total caloric depriviation --Tissue/muscles wasting (broomstick extremities) -loss of sub q fat (decreased leptin-->in increased cortisol-->lipolytic agent) -variable edema
|
|
|
Vitamin A deficiency |
Night blindness dry, scaly skin (xerosis cutis) hair loss corneal degernation (keratomalcia) immune suppression
|
|
|
What does vitamin A toxicity present as? |
-suspect in women taking accutane for acne Arthraligias Cerebral edema PSEUDOTUMOR CEREBRI! osteoporosis hepatic abnormaities
-Clef palate and cardiac abnormalities in fetus |
|
|
Thiamine deficiency |
B1=Thiamine
--Decreased glucose breakdown-->ATP depletion worse if give glucose (why give suspected alcoholics thiamine before glucose if present to ER). -Dry Beriberi: peripheral neuropathy (demylination) -Wet Beriberi: tachy (& lots of other HF signs), constipation and neuro symptoms Wernicke-Korsakoff: 1. Confusion 2. opthalmoplegia, 3 Ataxia + personality change and difficultying talking, permanent memory loss
|
|
|
Pellegra |
Severe deficiency of B3 (Niacin)
Decreased tryptophan absorption from a corn based diet (think deep south)
-Diarrhea -Dementia (& hallucinations) -Dermatitis (on places where the sun shines) |
|
|
What are causes of B3 deficiency? |
B3=Niacin -Hartnup disease (decreased tryptophan absorption) -Malignant carcinoid syndrome (increased tryptophan metabolism) -isoniazid (decreased vitamin b6) -Corn based diet (low in tryptophan and niacin) |
|
|
How would B6 deficiency present? |
B6=pyridoxin
Convulsions Hyperirritability Peripheral neuropathy Sideroblastic anemias (impaired hemoglobin synthesis and iron excess-->trapped in mito!) |
|
|
How would B9 deficiency present |
B9=FOLIC ACID!! -Tricky....
Macrocytic, megaloblastic anemia with hypersegmented PMNS |
|
|
What are the labs associated with B9 deficiency? |
B9=Folic acid
Increased homocystine normal methymalonic acid |
|
|
What are the labs associated with B12 deficiency? |
Increased homocystine Increased methymalonic acid |
|
|
How does vitamin c toxicity present? |
N/V/D Fatigue Calcium oxalate nephrolithiasis (increased risk of iron toxicity in some pts (transfustions, hereditary hemochromatosis) |
|
|
What is the association between sarcoidosis and pts having gallstones, bone pain and abdominal discomfort? |
Sarcoid has non-caseating granulomas -epitheliod cells in granuloma increase the activation of vitamin D--->increases absorption of calcium
Hypercalcemia=stones bones and moans |
|
|
How does Vit E deficiency present? |
Hemolytic anemia Acanthocytosis (spur cells or spiculated RBCS) Muscle weakness Posterior columbn and spinocerebellar tract demyelination |
|
|
What are the signs of zinc deficiency and who do they usually present in? |
-Alcoholics, DM, chronic diarrhea -DELAYED WOUND HEALING -can't taste (dysgeusia) -can't smell (anosmia) -decreased adult hair |
|
|
How does copper deficiency present? |
Microcytic anemia (binds iron to transferrin) Aortic dissection Poor wound healing (cross links collagen and elastic tissue)
|
|
|
What has the highest death rate of psychiatric disorders? |
Anorexia nervosa --death from laxative abuse-->hypokalemia-->arrythmias--->sudden death
Laxative abuse-->cause of non-ion gap metabolic acidosis |
|
|
Clinical findings of an anorexia nervosa patient |
-secondary amenorrhea (decreased GnRH due to excessive loss of body fat/weight)-->decreased Lh & FSH-->decreased estradiol -Osteroporosis (less estrogen-->decreased osteoblast activity and increased osteoclast activity) -Euthyroid sick syndrome--> decreased T3-->bradycardia, hypotension, cold intolerance, skin/hair changes (yellow skin from increa carotene due to decreased metabolism) -Increased GH and cortisol |
|
|
How do you treat anorexia nervosa? |
Cognative behavioral therapy
SSRIs |
|
|
Clinical findings of Bulimia nervosa |
-dehydration -Face/neck swelling -Russell sign (bruises on back of hands from self inducing vomiting) -hematemesis from tears/rupture of distal esophagus -vomiting-->hyponatremia, hypokalemia, metabolid alkalosis (loss of stomach acid)-->arrhythmias-->sudden death -laxative abuse-->non ion gap metabolic acidosis -Electrolyte abnormalities |
|
|
Specialized diets for DM |
-Limit carb intake-->substitute with monounsaturated fats (olive oild, canola oil, nuts, avocados)
Big picture is to decrease TG and increase HDL and limit cholesterol to 300mg daily
-Try to loose weight by eating fewer calories |
|
|
Special diets for renal failure |
-Restrict protein (to decreased urea nd ammonia production) -Limit fluids -limit salt, potassium, phosphorus, and other electrolytes needed to be filtered by kidney |
|
|
Transfusion Related Acute Lung Injury (TRALI) |
-Noncardiogenic pulmonary edema caused by passive transfer of donor antineurtophil or anti HLA antibodies into the recipient -antibodies bind and activate recipient neutrophils in the lung-->increased vascular permeability and pulmonary capillary leak (ARDS)
Sx begin within 6 hours of transfusion -Tx: is supportive & STOP TRANSFUSION |
|
|
Transfusions associated circulatory Overload (TACO) |
Caused by hypervolemia from administration of blood products and IV fluids -SOB, cough, pulmonary and peripheral edema tx: dieuretics/fluid restriction
No contraindication of future blood products
|

