![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
742 Cards in this Set
- Front
- Back
|
What is the absolute refractory period of cardiac muscle?
|
250ms
|
|
|
What is the approximate delay in the AV node?
|
0.1s
|
|
|
Cardiac output (CO) is...
|
The amount of blood pumped per minute.
HRxSV |
|
|
Cardiac reserve is...
|
The difference between resting and maximal CO.
|
|
|
How do we calculate stroke volume?
|
EDV-ESV
EDV = amount of blood collected in a ventricle during diastole. ESV = amount of blood remaining in a ventricle after contraction. |
|
|
What three factors affect stroke volume?
|
Preload
Contractility Afterload |
|
|
How do exercise and slow heart rate increase stroke volume?
|
Increased venous return and improved ventricular filling.
(Frank-Starling) |
|
|
What is contractility?
|
Contractile strength, independent of stretch and EDV.
|
|
|
What increases contractility?
|
Increased sympathetic stimuli
Certain hormones (eg. epi) Ca2+ and some drugs |
|
|
What decreases contractility?
|
Acidosis
Increased extracellular K+ Calcium channel blockers/Beta bockers |
|
|
How is striated muscle (skeletal and cardiac) organized?
|
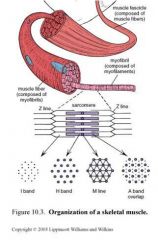
|
|
|
Why do muscle cells have a striated appearance?
|
Myofilaments, the critical contractile elements are in register with each other.
|
|
|
What are the three troponins invovled in muscle contraction?
|
Troponin-T: binds to tropomyosin, anchoring the troponin complex
Troponin-C: binds Ca2+, an essential step in initiating contraction Troponin-I: binds to actin, inhibiting actin-myosin interaction. |
|
|
What does tropomyosin do?
|
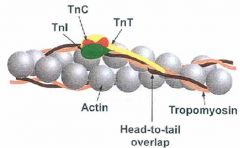
Tropmyosin holds F-actin (a polymer of G-actin) together.
The entire structure is called a thin filament. |
|
|
What is the preferred enzyme test for myocardial injury?
|
Cardiac isoforms of troponin T and I (cTnT and cTnI)
Rise 2 - 6 hours after injury. Peak 12 - 16 hours cTnI elevated for 5-10 days. cTnT elevated for 5-14 days |
|
|
Thick filaments are made of Myosin II. What is Myosin II made out of?
|
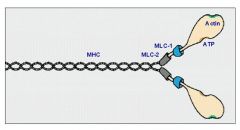
Each myosin II molecule contains two polypeptide heavy chains and four light chains (two each of two types).
Each myosin heavy chain (MHC) has globular head with one binding site for ATP and another for actin; also demonstrates ATPase and motor activity. Myosin light chains (MLC) play structural and regulatory roles. |
|
|
What do the filaments bind to in the structure of a sarcomere?
|
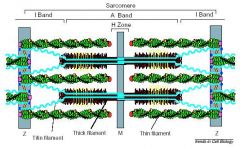
Thick filaments bind to M lines.
Thin filaments bind to Z lines. |
|
|
What are the stages of the muscle contraction cycle on the level of actin and myosin?
|
Attachment (rigor formation)
Release Bending Force generation Attachment: The Sequel |
|
|
What actually causes shortening of the muscle cell?
|
Rapid contraction cycles that move the thin filaments along the thick filaments.
|
|
|
What role does ATP play in muscle contraction?
|
When ATP is absent the myosin head is tightly bound to actin.
(called “rigor configuration” because it causes rigor mortis in the complete absence of ATP). Binding of ATP to the myosin head causes conformational changes in the actin binding site and reduces the affinity of the myosin head for the actin molecule. The head then uncouples from the thin filament. The Myosin head then undergoes further conformational changes, initiated by the breakdown of ATP, which cause it to bend. (Mg++ is a critical cofactor for this ATPase). The Myosin head then binds weakly to new binding site on a neighboring actin molecule if Ca2+ is present. Phosphate is released and the binding affinity between myosin and actin then increases. The force is generated by the myosin head as it returns to its unbent position because it forces movement of the thin filament along the thick filament.During this “power stroke” ADP is lost from the myosin head. With loss of ADP the myosin head is again left attached to the thin filament (rigor configuration) but to a different actin molecule, one closer to the Z line. If ATP still available the cycle can repeat. |
|
|
What do we see when we look at contraction at the level of the sarcomere? (sliding filament model)
|
I band and H band narrow but the A band maintains its width.
|
|
|
What are the key regulators of muscle contraction?
|
ATP
Calcium Mg Na beta adrenergic receptors. |
|
|
What is the role of the sarcolemma and the transverse tubules?
|
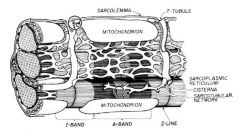
The sarcolemma conducts AP quickly over the surface of the muscle fiber.
T tubules are continuous with the sarcolemma and transduce the action potential into the sarcoplasmic reticulum which has stores of calcium to initiate contraction. |
|
|
How is excitation/contraction coupling different in cardiac muscle than in skeletal muscle?
|
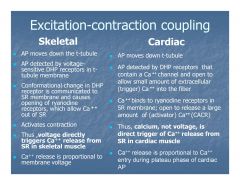
In skeletal muscle release of Ca++ from the sarcoplasmic reticulum is directly voltage dependent.
In cardiac muscle release of Ca++ from SR is Ca++ activated (calcium-dependent calcium release). |
|
|
How is an action potential propagated in cardiac muscle different from one propagated in a neuron?
|
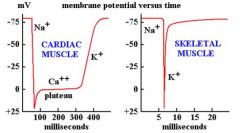
Both are about 100 mV
But DURATION of cardiac muscle AP is two orders of magnitude longer than in nerve cell or skeletal muscle. (cardiac AP ~ 300 ms; Nerve cell AP ~3ms) |
|
|
Why happens to skeletal muscle as it ages?
|
It starts to increasingly resemble cardiac muscle.
|
|
|
Compared to skeletal muscle how is cardiac muscle structurally different?
|
Contains more and larger mitochondria.
Contains more glycogen granules. Contains larger and more numerous T tubules (in ventricular muscle). Contains multicellular “fibers” that are electrically and mechanically coupled via intercalated disks. Intercalated discs cross the muscle cells transversely (parallel to the striations). |
|
|
Activity initiated in a cardiac muscle cell propagates in which direction?
|
Any direction.
Except at the boundary between the atria and the ventricles because of the presence of non conducting fibrous tissue. |
|
|
What does each intercalated disc contain?
|
Transverse components visible with light microscopy and
lateral components which are not evident with light microscopy. Fascia adherens- Anchor for thin filaments of terminal sarcomere. Major transverse component. Maculae adherens (desmosomes)- Prevent cells from pulling apart under tension. Transverse and lateral. Gap junctions (communicating junctions)- Major lateral component. Create ionic continutity and permit cardiac muscle cells to behave as a syncytium. |
|
|
How much faster than regular cardiac muscle fibers does the conduction system conduct impulses?
|
4x
|
|
|
What are the cellular characteristics of the SA node?
|
Smaller than other cardiac cells.
Fewer and more poorly organized myofilaments. Lack intercalated disks but have some intercellular junctions. !!Slow SPONTANEOUS depolarization in the late stage of each action potential!! Isolated nodal cells depolarize 80-100 /minute. (faster than normal HR because of denervation). Fastest cell will control the rest in SA node. |
|
|
What does the SA node look like histologically?
|
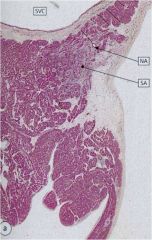
Embedded within fibrocollagenous supporting tissue.
Fibers fuse with surrounding atrial muscle fibers. Connected to internodal fiber bundles which run to the AV node. |
|
|
At what rate does conduction occur in the atria?
|
0.3 m/s
|
|
|
At what rate does conduction occur in the internodal fibers?
|
1 m/s
|
|
|
Roughly how long is the AV node delay?
|
0.1 s
|
|
|
What is the intrinsic rate of the AV node?
|
40-60
Should not autodepolarize unless SA node or internodal fibers are dysfunctional. |
|
|
What is the conduction rate in purkinje fibers?
|
~0.3-0.5 m/s
|
|
|
What are the histological characteristics of purkinje fibers?
|
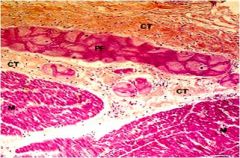
Large size
Abundant glycogen Do not contain large amounts of contractile fibers so not as eosinophilic as cardiac muscle cells. Frequent gap junctions/infrequent intercalated discs. Insulation by sheath of connective tissue. |
|
|
How does the duration of the action impulse compare between the epicardium and endocardium?
|
Duration is much shorter near the epicardium so the termination of activity appears as if it were propagating from the epicardium toward the endocardium.
|
|
|
What is the total time from SA node firing to distribution of the impulse along the entire heart?
|
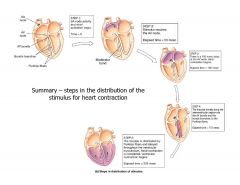
~225 ms
|
|
|
How long does the entire cardiac cycle take?
|
~0.8s
|
|
|
What is the average stroke volume?
|
~70cc
|
|
|
What percentage of patients with MI die before they reach the hospital?
|
~1/3
|
|
|
What ions cause depolarization to occur?
|
Calcium induced sodium driven depolarization.
Calcium. Potassium repolarization. |
|
|
What direction does contraction occur in ?
|
A clockwise ringing type motion from the apex to the base of the heart.
|
|
|
What proportion of the cells in the heart are fibrous (non muscle)?
|
~ 2/3
|
|
|
How does a propagating sodium channel impulse induce contraction?
|
The propagating sodium current hits an L-type calcium channel at the bottom of the invagination into the myocyte.
This open a gate that allows a small amount of calcium to enter the cell and bind the ryanodine receptor channel on the sarcoplasmic reticulum. The SR then releases a large amount of calcium into the cytoplasm. The calcium causes contraction. |
|
|
In general, electrical conduction always preceeds...
|
Mechanical contraction.
|
|
|
How long is atrial systole?
|
0.1 s
|
|
|
How long is ventricular systole?
|
0.4 s
|
|
|
How long is ventricular diastole?
|
0.4s
|
|
|
What is a normal end diastolic volume?
|
~135 cc
|
|
|
What is a normal end systolic volume?
|
~ 65cc
|
|
|
How do we calculate EF?
|
EF=SV/EDV
|
|
|
Cardiac output is a function of stroke volume and HR, what influences each?
|
HR by automaticity.
SV by contractility, afterload and preload. |
|
|
By what means does the central nervous system influence the heart?
|
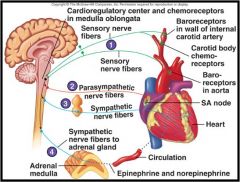
|
|
|
How does autonomic tone influence heart rate?
|
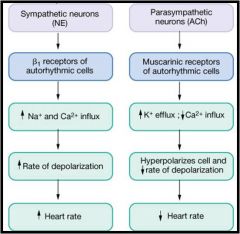
|
|
|
End diastolic volume can be though of as a measure of...
|
Preload
|
|
|
What increases preload?
|
Inspiration
Exercise Aortic Regurg Systemic Shunt |
|
|
What decreases preload?
|
Valsalva
Dehydration Atrial Fibrillation Medications (diuretics etc.) |
|
|
When we refer to afterload we are generally referring to....
|
Mean arterial pressure or Aortic Valve/ outflow tract resistance.
|
|
|
Increased afterload causes...
|
Longer isovolumetric contraction and slower rate of muscle fiber shortening.
|
|
|
How does contractility influence SV?
|
Increased contractility results in a greater stroke volume at any given preload or afterload.
|
|
|
One of the best ways we can measure contractility is the...
|
End systolic pressure volume ratio.
|
|
|
How do Epi and NE influence the myocardium?
|
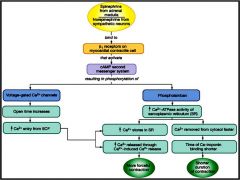
Inotropic
Chronotropic |
|
|
Under normal physiological conditions, excercise causes...
|
Rapid Relaxation
Heart fills under low pressure Stroke volume increases In contrast, when a person with CHF excercises there is: Slow Relaxation Heart fills under high pressure Stroke volume only has a small increase. |
|
|
People with CHF can generate more work with their myocardium but they do it at the expense of...
|
Higher end diastolic pressure.
|
|
|
CHF is characterized by increased...
|
Pressure in the left ventricle.
|
|
|
Why is a CHF a situation where the worse it gets, the worse it gets?
|
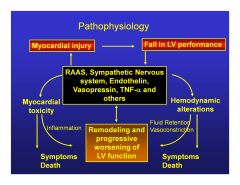
|
|
|
CHF pathophysiology can be thought of as an imbalance of...
|
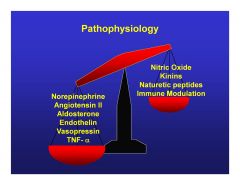
|
|
|
What are the three fates of chronic heart failure cells?
|
They can be signalled to...
Grow Become fibrotic Designate themselves for death. |
|
|
What are the thrre hemodynamic defense mechanisms that lead to worsening heart failure?
|
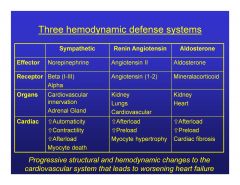
Sympathetic-Myocyte death
Renin angiotensin-Myocyte hypertrophy Aldosterone-Cardiac fibrosis |
|
|
According to the ACC/AHA, what are the four stages of CHF?
|
A. High Risk no structural changes
B. Structural changes without syndrome C. Syndrome controlled with therapy D. Syndrome uncontrolled despite therapy |
|
|
What are the NYHA classes of CHF symptoms?
|
I.
No limitation of activity II. Mild limitation activity improves by slowing down. III. Marked limitation improves with rest. IV. Severe limitation or symptoms at rest. |
|
|
What type of hypertrophy do we find in systolic heart failure?
|
Eccentric hypertrophy.
|
|
|
What type of hypertrophy do we find in diastolic heart failure?
|
Concentric hypertrophy.
|
|
|
Near normal SV
Normal EDV Steep diastolic PV relationship is.... |
Diastolic heart failure
|
|
|
Low SV
High EDV Steep diastolic PV relationship is... |
Systolic heart failure.
|
|
|
What are the epidemiological differences between systolic and diastolic heart failure?
|
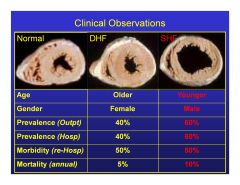
Younger males usually systolic HF.
Older females usually diastolic HF. |
|
|
How does the sarcoplasmic reticulum content of cardiac muscle compare to skeletal and smooth muscle?
|
SR is less abundant than in skeletal muscle, but greater in density than smooth muscle
!Sarcolemma has specialized ion channels that skeletal muscle does not – voltage-gated Ca2+ channels! |
|
|
A junctional rythm from the AV node would be at a rate of about...
|
55 bpm
|
|
|
What are five causes of heart failure?
|
Pump failure.
Obstruction to flow. Regurgitant flow. Disorders of Conduction. Disruption of the continuity of the circulatory system. |
|
|
What is the ideal sarcomere length?
|
2 - 2.4 micrometers
|
|
|
How do ventricular pressure curves work?
|
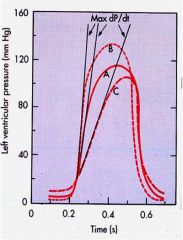
A hypodynamic heart has lower dP/dt, higher diastolic pressure, and reduced ejection time (C).
A hyperdynamic heart has lower diastolic pressure, higher dP/dt, brief ejection phase (B). |
|
|
How do pressure/volume loops work?
|
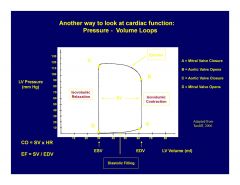
Starting at the Southeast corner and working Counterclockwise:
Mitral valve closure.(point)=EDV Isovolumic contraction.(line) Aortic valve opening.(point) Ejection.(line) Aortic valve closure.(point) Isovolumic relaxation.(line) Mitral valve opening. (point)=ESV Diastolic filling (line). Repeat. Rate of change (dP/dV) at aortic valve closure (NW corner) is a measure of contractility. |
|
|
What is LV ESPVR?
|
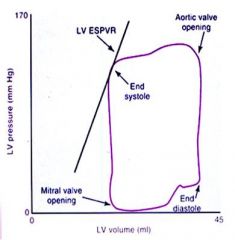
The slope of the line represents left ventricular end-systolic pressure volume relationship and is an indicator of inotropy.
Contractility is improved with inotropic drugs and the slope is moved up and to the left. Ischemia reduces blood flow, decreasing myocardial contractility. The slope therefore moves down and right. |
|
|
What changes in ion concentrations occur in a cardiac muscle fiber following depolarization?
|
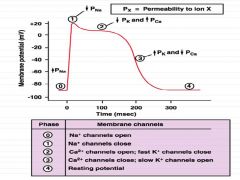
Phase 0: Na+ channels open
Phase 1: Na+ channels close Phase 2: Ca++ channels open; fast K+ channels close Phase 3:Ca++ channels close; Slow K+ channels open. Phase 4: Resting potential |
|
|
What is Poiseuille’s Law ?
|
For a steady state, laminar flow newtonian fluid through a cylindrical tube, the flow (Q)
varies directly as the pressure difference (P1 - P0) and the fourth power of the radius, (r) for the tube, and it varies inversely with the length (l) of the tube and the viscosity (n) of the fluid. Q= p(P1 - P0) r^4/8nl In humans the principal resistance to flow is lumen caliber. |
|
|
SVR = BP/CO which means that...
|
BP=CO*SVR=(MAP-CVP)/(CO x 80 dynes x sec x cm-5)
|
|
|
During pregnancy BP...
|
Decreases.
The fetal circulation is a low resistance circuit. |
|
|
Small boxes on ECG strips are...
|
0.04s
Large boxes are 0.2s (300,150,100,75,60,50) |
|
|
Purkinje cells are found on the border between the...
|
Endocardium and myocardium
(larger cells with pale staining centers because of glycogen stores) |
|
|
What are the valves made of ?
|
They are extensions of the endocardium.
Leaflets of collagenous tissue covered by endothelium are built around a CENTRAL FIBROUS SHEET(lamina fibrosa). Spongiosa is on the atrial or blood vessel side of each valve. Ventricularis is on ventricular side. !!!NO MUSCLE!!! |
|
|
What is the histological difference between endocardium and epicardium?
|
Both endocardium and epicardium have simple squamous epithelium on the surface.
Only the epicardium though contains nerve bundles, larger blood vessels and large fat deposits. |
|
|
Why is it that when we destroy cardiac muscle cells we cannot regenerate them?
|
Unlike skeletal muscle which has sattelite cells, the myocardium does not have progenitor cells.
|
|
|
What is contained in the endocrine granules of atrial cardiac muscle cells?
|
Atrial natiuretic hormone.
Released when atrial fibers are excessively stretched. Increases excretion of water, Na and K by the kidney. Also inhibits renin and aldosterone to lower BP. More of these endocrine granules are found in the RA than LA. |
|
|
What are the three layers of a blood vessel?
|
Tunica intima – closest to lumen; endothelial cells oriented along direction of blood flow.
Tunica media – middle layer with elements oriented circumferentially Tunica adventitia – outermost layer with elements ordered longitudinally. |
|
|
What is the tunica intima made of ?
|
Endothelium with basal lamina.
Subendothelial connective tissue (particularly in elastic vessels near the heart.) |
|
|
What is the tunica media made of?
|
Smooth muscle.
Variable amount of elastin and collagen. Dominant layer in arteries. |
|
|
What is the tunica adventitia made of?
|
Collagenous fibers and a few elastic fibers.
Major component in veins, with significant smooth muscle. Contains vaso vasorum and nervi vascularis in larger vessels |
|
|
What factors are secreted by endothelium?
|
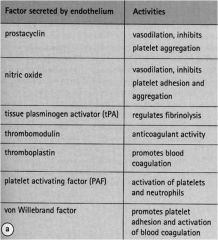
Prostacyclin
Nitric oxide tPA Thrombomodulin Thromboplastin PAF vWF |
|
|
What do atherosclerotic plaques do to the relationship between endothelium and the intima?
|
Foam cells and friends create a spatial seperation which prevents endothelial cells from regulating smooth muscle.
|
|
|
What are arterioles?
|
Smallest branches of the arterial tree.
Start of the microvascular system. 30 to 400 micrometers in diameter. Intima of endothelial cells with basement membrane. Media with 1-2 layers of smooth muscle cells; as the arterioles become smaller the smooth muscle cells become increasingly discontinuous. Adventitia is insignificant. Regulate vascular resistance and flow into and through capillary beds. |
|
|
Why are postcapillary venules important?
|
Primary site of migration of leukocytes out of blood stream.
Regulated by factors produced by endothelial cells (e.g., selectins, integrins) and factors produced by cells outside of blood vessels (e.g., interleukins). |
|
|
What are the differences between medium arteries and veins?
|
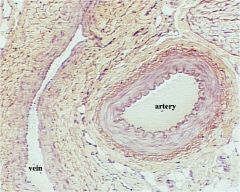
|
|
|
At any given time, how much blood is in the venous system?
|
about 65-70%
This is sometimes called capacitance. |
|
|
What are pericytes?
|
Cells which are unique to capillaries and found adjacent to endothelium.
Contractile proteins within the pericytes provide some regulation of capillary diameter. |
|
|
How does transport occur in capillaries?
|
The endothelial cells for tight junctions so most transport is done through the endothelium by pinocytotic vesicles.
(continuous capillaries found in muscle nerve and connective tissue) |
|
|
What is a fenestrated capillary?
|
Contain fenestrae, bridged by diaphragms of extracellular material.
Found in the pancreas, intestines, endocrine glands, and renal glomeruli (where they lack diaphragms). The basal lamina remains continuous but inner surface looks bumpy. |
|
|
Skeletal muscle is characterized by...
|
peripherally located nuclei.
|
|
|
What is a sinusoid?
|
Have large fenestrae without diaphragms, gaps between endothelial cells, and a scanty, discontinuous or absent basal lamina.
Bigger than capillaries and found in regions that require a lot of transport like the bone marrow, liver, spleen, and lymphoid organs. They have irregular shapes. |
|
|
What is the purpose of AV shunts in the skin?
|
Opening the AV sphincters (under CNS control) diverts blood to the deeper shunts thus conserving heat.
Closing the shunts diverts blood to the capillary bed thus dissipating heat. |
|
|
What are the characteristics of lymphatic capillaries?
|
Single layer of endothelial cells with no fenstrae or tight junctions and an incomplete basal lamina.
Endothelial cells overlap but have intercellular spaces that facilitate vessel permeability. Bundles of lymphatic anchoring filaments terminate on the abluminal plasma membrane and maintain patency of the flimsy vessels. |
|
|
Large lymphatic vessls can be recognized by the fact that...
|
Their tissues are not as well organized into layers as veins and arteries.
|
|
|
How do we define atherosclerosis?
|
A variable combination of changes in the intima
of arteries, consisting of focal accumulation of lipids, cells, fibrous tissue, complex carbohydrates, blood, blood products and calcium, and associated medial changes with progression of disease. |
|
|
What is arterioscleorsis?
|
A more general term than atherosclerosis which refers to any proliferative or degenerative stiffening of arteries.
|
|
|
Where are the sites with a higher predilection for atherosclerosis?
|
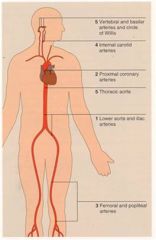
Proximal main coronary arteries.
Carotid arteries and circle of willis. (~ 10 years after coronaries) AA and iliac arteries (more prevalent in smokers) Femoral and popliteal arteries. (more common in smokers) |
|
|
The first abnormal macroscopic (usually forensic) finding in athersclerosis is...
|
Fatty streaks.
Macrophage foam cells that have ingested cholesterol ester droplets. Fatty streaks progress more rapidly in men than women. |
|
|
What is the approximate size of molecule that can get through endothelial lining?
|
<40,000 MW
Proteins and lipoproteins should not get in but they are sometimes taken up by the non receptor mediated process transcytosis. (strictly concentration dependant). |
|
|
What cell types are involved in formation of an atherosclerotic plaque?
|
Endothelial cells:
Endothelial dysfunction occurs early but actual desquamation (loss) does not occur until later. Macrophages: Early and Late T-cells: Early and late, but in smaller numbers than macrophages. Smooth muscle cells: Accumulate in intima at a later stage than macrophages. Mast cells: Later (minor component) Neutrophils: Not usually found in atherosclerotic plaques, except following plaque rupture. |
|
|
Where do we see an increase in cholestrol ester in atherosclerotic plaques?
|
Intracellular first.
Extracellular lipid deposition is later. |
|
|
What role does connective tissue (fibrous tissue) play in the formation of plaques?
|
Collagen:
Tensile strength Synthesis is stimulated by SMCs of Athero. Plaques Elastin: Provides elasticity to the arterial wall. Proteoglycans: Binds LDL/VLDL in sub-endothelial space, which increases retention and facilitates oxidation, etc. |
|
|
Besides lipids and fibrous tissue, what other components are involved in plaque formation?
|
Calcium and some magnesium
Bone formation Blood products (old fibrin or new thrombi) |
|
|
So how does the pathogenesis of athersclerosis actually begin?
|
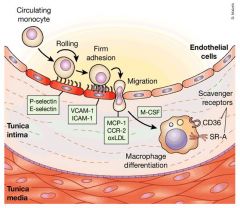
Concentration dependant transcytosis of lipoproteins into the sub-endothelial space of the arterial wall, retention of LDL/VLDL by matrix.
Proteoglycans, expression of monocyte adhesion molecules on the endothelial cells. Recruitment of monocytes into the artery wall and their differentiation into macrophages. These macrophages then begin to express scavenger receptor A and CD36 that recognize abnormal lipoprotein made as a result of oxidation or other mechanisms. The macrophages then engulf these abnormal lipoproteins and become foam cells. |
|
|
How do Foam Cells form once macrophages begin to accumulate in the sub-endothelium of the arterial wall?
|
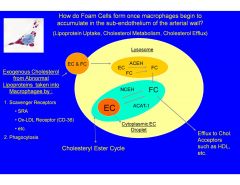
The cholesterol that cannot be converted to free cholestrol and transported out by HDL remains as esterified cholestrol droplets in the cytoplasm.
|
|
|
What is the only risk factor that by itself will cause atherosclerosis?
|
Hypercholesterolemia
|
|
|
What is the benefit of lowering Plasma Lipoprotein concentrations?
|
Lipid lowering not only reduces the transcytosis of plasma lipoproteins into the arterial wall, which reduces the development of foam cells, it also acts as an anti-inflammatory intervention, by decreasing the number of macrophage foam cells and T-cells, and increasing connective tissue synthesis. This is known as plaque remodeling. The result is a more stable plaque, less susceptible to rupture.
|
|
|
How does plaque rupture occur and how does it lead to thrombosis and the Acute Coronary Syndrome?
|
Macrophage foam concentrate at the unstable shoulders of the plaque and secrete matrix metalloproteinases (MMPs).
MMP's break down collagen. Rupture of the plaque interrupts the endothelial surface which exposes blood platelets to underlying collagen and to macrophages that also secrete tissue Factor. This causes platelet aggregation which initiates thrombosis. |
|
|
What determines whether or not a plaque is unstable?
|
The amount of collagen on the edges.
Lots of collagen, less likely to rupture. |
|
|
HRT in women who have not had a hysterectomy MUST include...
|
Progesterone along with the Estrogen.
|
|
|
What is the advantage of conjugated equine estrogen at time of menopause?
|
CEE reduces CHD in women who have hada hysterectomy if given AT THE TIME of menopause (~51).
If given later it increases the risk of stroke and CVD. |
|
|
What proportion of circulating cholestrol comes from biosynthesis?
|
~80%
|
|
|
What is the relationship between risk of CHD and LDL?
|
log linear.
For every 30 mg/dL change in LDL-C, the relative risk for CHD is changed by ~30%. This extends down to about 40mg/dL. |
|
|
How is HDL Anti-atherogenic ?
|
Mediates cholesterol efflux (via binding to macrophage foam cell ABCA1 and ABCG1 transporters) from atherosclerotic plaques and other tissues which is the first step in reverse cholesterol transport.
Anti-inflammatory by reducing expression of adhesion molecules (p-selectin, VCAM-1, etc.) on endothelial cells. Stimulates nitric oxide production (eNOS) in endothelial cells which stimulates vasodilation. Anti-oxidant properties. |
|
|
How does smoking increase the risk of CHD?
|
Increased atherosclerosis.
Increased chance of sudden death (acute thrombotic event). This is the major effect. Cessation of smoking reduces the risk of CHD to nearly that of a nonsmoker in < 3 years. |
|
|
The primary risk factors for CHD include....
|
Elevated Plasma Chol. (LDL)
Low HDL Hypertension Cigarette Smoking Diabetes Mellitus |
|
|
The secondary risk factors for CHD include...
|
Obesity
Sedentary life style Type A personality etc. |
|
|
Flow in a vessel is given by...
|
Delta P / resistance
|
|
|
Resistance is approximately given by..
|
(Vessel length*Blood viscosity) /r^4
ή * L/r^4 The radius is therefore by far the biggest factor influencing vascular resistance. |
|
|
Poiseuille’s equation for a single vessel is derived from flow in a vessel and resistance in a vessel. What is it?
|
Flow ~ (ΔP * r^4)/ (ή * L)
|
|
|
The vessels that have the largest impact on peripheral resistance in a series arrangement are...
|
The arterioles.
|
|
|
For vessels in a parallel arrangement, we calculate resisatnce using...
|
An ohms law relationship.
|
|
|
The sum of all the peripheral resistance in the body excluding the pulmonary resistance is called...
|
Systemic vascular resistance or total peripheral resistance.
SVR and therefore BP is primarily regulated by changing the diameter of the precapillary arterioles. |
|
|
How do we calculate SVR?
|
SVR= (MAP-CVP) / CO
SVR is CALCULATED this way but not determined by these parameters. |
|
|
Precapillary arterioles by default have a certain amount of tone to them generated by vascular smooth muscle. How is this tone and thus their diameter regulated?
|
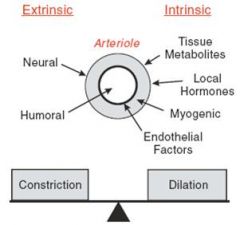
Extrinsically (Innervation and hormones-neurohumoral control)
Intrinsically (local factors like tissue metabolites, local hormones, myogenic tone and endothelial factors) |
|
|
What Autonomic CNS elements regulate the cardiovascular system?
|
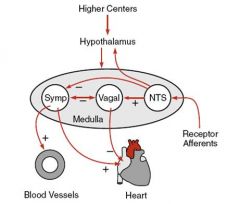
Medulla:
contains cell bodies for parasympathetic (vagal) and sympathetic efferent nerves Hypothalamus: modulates medulla activity during exercise or temp regulation. Higher centers (cortex): modulate cardiovascular function at times of emotional stress (fear, anxiety) |
|
|
What are the charcteristics of the parasympathetic nervous system?
|
Preganglionic fibers are long:
Preganglionics synapse in or near organ. Neurotransmitter is ACh. Receptor is nicotinic. Postganglionic fibers are short: Neurotransmitter is ACh. Receptors are muscarinic. |
|
|
What are the characteristics of the sympathetic nervous system?
|
Sympathetic ganglia are near spinal cord.
Preganglionic fibers are short: Neurotransmitter is Ach Receptors on postganglionic fiber are nicotinic. Postganglionic fibers are long. Neurotransmitter is NE. Receptors on tissues are alpha and beta adrenergics. |
|
|
The left vagus stimulates..
|
the AV node
Causes AV block. |
|
|
The right vagus stimulates...
|
The SA node.
Causes decreased firing rate. |
|
|
What happens during concurrent stimulation of the sympathetic and parasympathetic nervous systems?
|
The parasympathetic predominates.
|
|
|
What happens with concurrent sympathetic & parasympathetic blockade as in coadministration of atropine and propranolol?
|
Intrinsic heart rate.
|
|
|
What are the characteristics of the neural control of the cardiovascular system?
|
Rapid response.
Relatively constant blood pressure via feedback systems (reflex control). Global as well as discrete control of various regional circulations. Coordination of circulatory control with movement and emotional states. |
|
|
What are the important reflex pathways associated with the cardiovascular system?
|
Arterial Baroreflex
Cardiopulmonary Baroreflex Chemoreceptor Reflex |
|
|
Where are the arterial baroreceptors located?
|
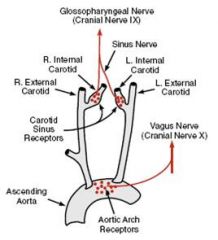
Carotid sinus
Aortic arch |
|
|
Where do the afferent fibers from the carotid sinus and aortic arch baroreceptors (stretch receptors) project to?
|
The nucleus solitarius.
|
|
|
What are the baroreceptors in the carotid sinus and aortic arch sensitive to?
|
Mean pressure.
Pulse pressure (i.e. SP-DP). Rate of change of pressure (dP/dt). |
|
|
What is the relation between arterial BP (baroreceptor input) & sympathetic nerve activity?
|
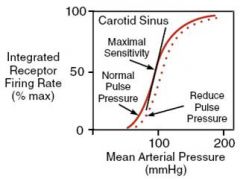
Sigmoid.
Mid-point of curve near prevailing BP (set point) Maximum sensitivity at mid-point of curve. Small deviations from the setpoint result in large changes in nerve firing rates. |
|
|
What are the two centers in the medulla that govern the sympathetic nerve activity?
|
Depressor center = caudal ventrolateral medulla (CVLM)
(Activation decreases SNA and BP) Pressor center = rostral ventrolateral medulla (RVLM) (Activation of this center increases SNA and BP.) The CVLM inhibits the RVLM. |
|
|
Increased baroreceptor input results in...
|
decreased SNA
increased PSNA |
|
|
Increased baroreceptor input results in...
|

decreased SNA
increased PSNA |
|
|
What are the cardiopulmonary baroreceptor reflexes?
|
Stretch receptors located in the heart respond to atrial filling (stretch); increased volume leads to sympathetic activation.
Increased blood volume and venous pressure activate other receptors that inhibit vasopressin release (causing diuresis). |
|
|
What is the purpose of chemoreceptors?
|
The primary purpose is to regulate respiratory activity to maintain normal pH, pO2 and pCO2.
Secondary purpose is to affect CV function via medullary centers. |
|
|
Where are the chemoreceptors located?
|
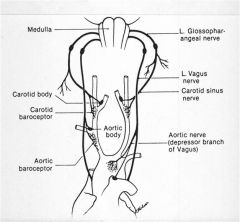
Aortic bodies
Carotid bodies |
|
|
What stimulates the carotid and aortic bodies?
|
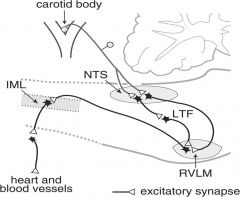
Increased CO2
Acidosis Decreased O2 Also stimulated by hypoperfusion. Ordinarily these structures have more blood flow/g tissue than any other. |
|
|
Where are catecholamines made?
|
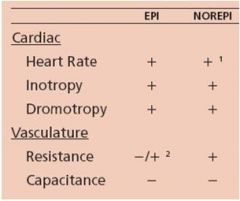
Released by adrenal medulla in a ratio of 80% Epi and 20 % NE in response to stress.
NE is also released by sympathetic nerve activation at the blood vessel. Noerpinephrine stimulates renin release and subsequently AngII and aldosterone. |
|
|
How does the renin angiotensin system work?
|
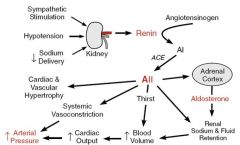
|
|
|
What is vasopressin and what stimulates it's release?
|
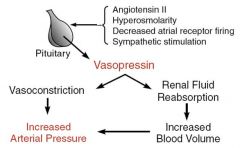
Released by the posterior pituitary.
Acts on the kidneys to increase blood volume and constricts blood vessels. Important for long term blood pressure control. Stimulated by: Angiotensin II. Hyperosmolarity Decreased atrial receptor firing. Sympathetic stimulation. |
|
|
What is atrial natiuretic peptide?
|
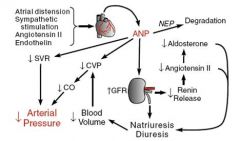
Peptide synthesized, stored and released by atrial myocytes in response to distention, Ang II, endothelin and sympathetic stimulation.
Responsible for long-term sodium and water balance, blood volume and arterial pressure. Counter-balances the RAAS. Used clinically as a marker of wall stress (eg. heart failure) |
|
|
What is vasodilator reserve?
|
The difference between basal and maximum flow.
|
|
|
What does A-V02 difference measure?
|
A-V O2 difference is a measure of oxygen extraction.
Small A-V O2 Diff. suggests additional oxygen can be extracted without greatly increasing flow. |
|
|
Which organ has the highest variability in blood flow? Which has the least?
|
Skeletal muscle ~2400%
Kidney ~ 16% |
|
|
Autoregulation of blood flow in organs occurs over a wide range of perfusion pressures, but has limits. What are those limits?
|
60-70 mmHg for maximal dilation and ~170 mmHg for maximal constriction.
|
|
|
What is the difference between active and reactive hyperemia?
|
Active hyperemia results from increased use.
Reactive hyperemia results from ischemia. |
|
|
Low oxygen concentration is generally a vasodilator, what is the exception?
|
The lung where low O2 is a vasoconstrictor.
|
|
|
What are some examples of endothelial factors that affect blood flow?
|
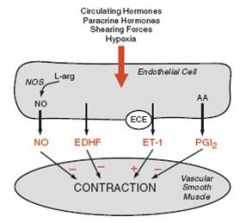
Nitric oxide and prostacyclin (dilators);
Endothelin, leukotrienes, thromboxanes (constrictors) |
|
|
What are the tissue vasodilator factors?
|
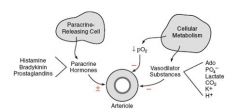
Phosphate
lactate CO2 K+ H+ Decreased PO2 |
|
|
What does shear stress do to the lumen of a vessel?
|
Causes the endothelium to produce NO and increases it.
|
|
|
What is the myogenic response of renal and GI blood vessels?
|
Originates from the smooth muscle (no neural input) in response to sudden pressure changes.
With increase in pressure, the smooth muscle contracts to preserve vascular resistance and vessel diameter. With decrease in pressure, the smooth muscle relaxes and the vessel dilates. |
|
|
What physical factors affect vascular resistance?
|
Temp.
Tissue compression. |
|
|
What is the difference between receptor dependant and receptor independant vasodilators?
|
Receptor dependant vasodilators affect the nitric oxide synthase process of converting L-arginine to L-citrulline.
Receptor independant vasodilators like nitroprusside are direct NO donors which can activate cGMP driven vasorelaxation in smooth muscle even if the endothelial cells are damaged (ie. angioplasty). |
|
|
What are the receptor dependant vasodilators?
|
Substance P
Histamine 5-HT BK AcH |
|
|
How do alpha 1 receptor agonists cause vasoconstriction?
|
They increase intracellular calcium in smooth muscle.
(Neuropeptide Y co-released with NE augments vasoconstriction action of NE) |
|
|
What endogenous vasoconstrictors act on vascular smooth muscle?
|
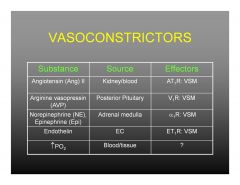
Angiotensin II
Arginine vasopressin NE and Epi. Endothelin Increased PO2 |
|
|
Where are the compressive forces on the coronaries highest during systole?
|
At the subendocardial level.
This makes it more vulnerable to ischemia because the subendocardium is fed EXCLUSIVELY during diastole. |
|
|
What sort of vasodilator reserve does the coronary circulation have?
|
Very high.
(80 ml/min/100g vs >400 ml/min/100g) Highly regulated by tissue metabolism especially adenosine which is a very strong vasodilator in the heart. |
|
|
In general, how do local factors interplay with autonomic innervation in the heart?
|
Local factors like metabolites tend to predominate.
|
|
|
What sort of oxygen extraction reserve does the heart have?
|
Very little.
Changes in oxygen demand must be met with changes in oxygen delivery. |
|
|
What constitutes the blood brain barrier?
|
Majority of capillary endothelium in the brain has tight junctions. It is surrounded by a basement membrane that is contiguous with astrocytes.
|
|
|
How is cerebral perfusion predominantly regulated?
|
!!! CO2!!!
Heavily relies on autoregulation. Adequate cerebral perfusion is also determined by systemic arterial pressure. |
|
|
What is the main autoregulatory metabolite of the coronary circulation?
|
Adenosine
|
|
|
What local factors are most important in regulating skeletal muscle perfusion?
|
Adenosine
K+ Hypoxia Innervation Local factors can overcome sympathetic innervation during a fight or flight response. |
|
|
Regulation of cutaneous circulation is predominantly...
|
Neural.
|
|
|
What happens to cutaneous circulation when we are hot?
|
Deep A-V anastomoses constrict and shunt blood towards the superficial "radiator" plexi.
Increased Core T means decreased cutaneous vascular resistance. |
|
|
What percentage of CO normally goes to the splanchnic circulation?
|
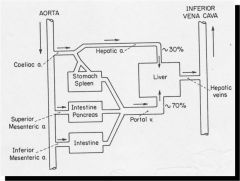
About 20%
contains ~15% of the circulating blood volume (BV). |
|
|
How is the splanchnic circulation controlled?
|
Mostly neural control of arterioles and veins.
Neurally released NE acts at a1 adrenergic receptors on VSM. High resting sympathetic VC tone; denervation or a1 adrenergic blockade increases splanchnic BF by ~1.5 times. |
|
|
What percentage of CO do the kidneys receive?
|
~20%
|
|
|
What is the difference between pulmonary and bronchial circulation?
|
Bronchial feeds the respiratory tissues whereas pulmonary is the systemic gas exchange circulation.
The bronchial circulation is derived from the aorta (left side). |
|
|
At what percentage of blood volume loss more or less do we start decompensating?
|
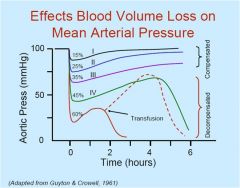
~40%
|
|
|
What are the compensation mechanisms for volume loss? (Hemorrhage)
|
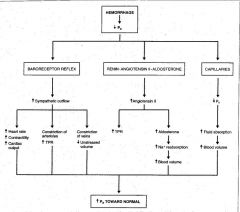
Short-term (seconds to minutes):
baroreflex, chemoreflex, ischemic response Intermediate (minutes to hours): renin angiotensin system, fluid shifts Long term (hours to days): renal salt and water conservation, new red cells, fluid replacement. |
|
|
What is claudication?
|
Leg pain with ambulation.
|
|
|
What are the hallmark signs of acute leg ischemia?
|
Pulselessness
Pain Pallor Poikilothermia (cold) Paresthesias Paresis/Paralysis (Always an emergency) |
|
|
A normal pulse on doppler is...
|
Triphasic
Biphasic or Monophasic (blunted, lacking flow reversal) |
|
|
Which skin changes are associated with arterial pathology?
|
Decreased temp
Pallor, mottling Hair loss Ulceration Gangrene |
|
|
Which skin changes are associated with venous pathology?
|
Edema
Varicosities Dermatitis (lipodermatosclerosis) Thickened skin Bronze discoloration Ulceration Gaiter distribution |
|
|
What is an antibrachial index?
|
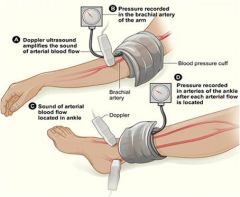
Opening pressures measured by Doppler, supine, at rest.
Highest of dorsalis pedis or posterior tibial in each leg divided by highest brachial artery pressure. Report a left and right leg but can use same arm pressure. |
|
|
What are normal values for ABI?
|
>1.0 Normal or possibly non-compressible from heavy calcification (e.g. diabetes)
<0.9 Abnormal, likely claudication (mean 0.6), marker of increased risk for heart disease. <0.6 Rest pain (mean 0.3) <0.3 Non-healing ulcers or gangrene. |
|
|
What is duplex ultrasonography?
|
Combines B-mode ultrasound & color Doppler with spectral analysis. Increased velocities and spectral broadening correlate with degree of stenosis.
Gold standard in screening for carotid occlusive disease and DVT. Used in renal, mesenteric, extremity arteries and bypass grafts to define stenoses. |
|
|
What does a DVT look like on duplex utrasonography?
|
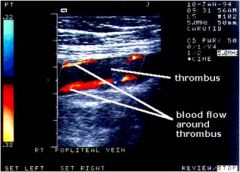
Noncompressible enlarged vein , abnormal flow, visible thrombus.
|
|
|
What does a normal Pulse-Volume Recordings and Segmental Pressures study look like?
|
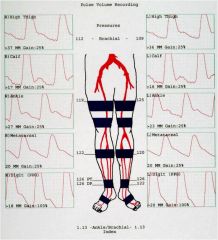
|
|
|
What does an abnormal (left sided occlusion) Pulse-Volume Recordings and Segmental Pressures study look like?
|
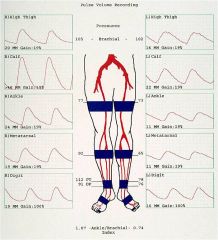
|
|
|
What is the advantage of digital subtraction angiography (DSA) for PVD?
|
Detailed lumen structure.
Invasive procedure though which involves radiation. Relatively contraindicated in kidney problems because of the dye. Can also cause drug reactions with metformin. |
|
|
What is the advantage of CT angiography for PVD?
|
Non-Invasive (radiation)
Contraindications Iodinated contrast renal insufficiency allergic reactions drug reactions Metformin |
|
|
Is MR angiography currently better or worse imaging than CT angiography?
|
Resolution of MR is worse.
|
|
|
What is the timeline of atherosclerosis?
|
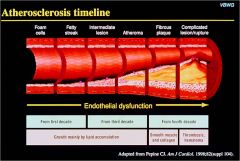
|
|
|
What are the major conclusions of the PDAY study about AAA?
|
The percentage of arterial surface with atherosclerotic lesions was the greatest in distal abdominal aorta (AA).
Total lesion area for the AA was greater in women than in men. Smoking selectively affects atherosclerotic changes in AA at a younger age than in the coronary arteries. |
|
|
How do we discern intermittent claudication from arthritis pain?
|
Occurs in muscle groups, not in joints.
Reproducible from one day to the next, and initially may become more noticeable while walking on incline. Goes away with relaxation. |
|
|
How do we tell pseudoclaudication from intermittent claudication?
|

Pseudoclaudication occurs with standing and variable walking distance.
Intermittent claudication does not occur with standing and usually within a fixed walking distance. |
|
|
What causes pseudoclaudication?
|
Spinal compression.
|
|
|
Regardless of patient age the most common cause of peripheral arterial disease over 40 is...
|
Atherosclerotic disease.
|
|
|
What is Buergers disease?
|
Thromboangiitis obliterans (Buerger's disease) is a cigarette smoking related vasculitis.
|
|
|
How do we differentiate between patients with Buergers disease and peripheral atherosclerosis?
|

|
|
|
How do we differentiate thrombosis from embolism?
|
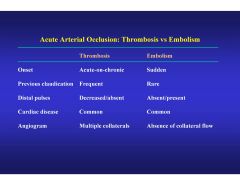
Acuity is the main factor as evidenced by onset and presence of collateral flow.
|
|
|
What are the independant risk factors for PAD in descending order?
|
Diabetes
Current smoking Age Hypertension Homocysteine Total cholestrol |
|
|
Which ORGAN is most likely to get atheromatous emboli?
|
kidney
|
|
|
What skin changes are associated with atheromatous embolization?
|
Blue toes
Livedo reticularis (net) |
|
|
What are the major collateral pathways in the lower extremities?
|
Lumbar aortic branches (Aortic disease)
Internal iliac system and pelvic circulation (CIA disease) Circumflex femoral arteries (CFA disease) Profunda femoris (SFA disease) Geniculate arteries (PA disease) |
|
|
Arterial occlusions generally occur at the ...
|
Bifurcations of arteries.
|
|
|
Typically the supply of oxygen to the tissues should occur at a ratio of...
|
2:1
|
|
|
Why does excercise cause a decrease in area pressure in chronic PAD?
|
Increased demand causes vasodilation of collateral vessls that are developed because of the occlusion.
|
|
|
PAD puts patients at greatly increased risk of subsequent...
|
MI
Stroke More PAD |
|
|
What is Leriche’s Syndrome?
|
Focal occlusive disease of the distal aorta and proximal iliac arteries.
|
|
|
What are the MOA's of the antiplatelet Rx?
|
|
|
|
The best risk reducing drug for PAD patients is...
|
Plavix.
Better than aspirin in CAPRIE study. |
|
|
What is Cilostazol?
|
Approved for claudication. (symptoms. Plavix for risk reduction)
Platelet aggregation inhibitor. !Vasodilator! phosphodiesterase III inhibitor which raises cGMP. !contraindicated in patients with heart failure! Best with smoking cessation and excercise. |
|
|
Flow times resistance (Q*R) is equal to...
|
Delta P
(BP = CO x SVR) Or BP = [LVEDV-LVESV] x HR x SVR |
|
|
Where are alpha 1 receptors predominantly found?
|
Vascular smooth muscle.
Stimulation causes vascular constriction (Pressor effect). |
|
|
Alpha 2 receptors are found in vascular smooth muscle at lesser density than Alpha 1 receptors but the main clinical use of Alpha 2 receptors is...
|
Stimulation causes negative feedback on presynaptic neurons.
The more we stimulate alpha 2 the less response we will have to other stimuli. Alpha 2 receptors in the brain also have roles in pain and sedation. |
|
|
What are the main effects of beta 1 stimulation?
|
Inotropic effect (cAMP)
Chronotropic effect – all tissues Lusitropic (relaxation) effect They also have a positive feedback effect on presynaptic receptors and increase the response to epi and NE. |
|
|
What is the normal Beta1:Beta2 ratio in the heart?
|
4-6:1
1-1.5:1 in CHF |
|
|
What is the main clinical use of Beta 2 receptors?
|
Bronchodilation (relaxation of bronchial smooth muscle).
They also produce a small amount of vasodilation. |
|
|
What is the main clinical use of dopamine (DA) receptors?
|
There are many dopamine receptors on the splanchnic vasculature and stimulation of these vasodilates and improves flow to gut, kidneys, liver etc..
There are relatively few in the heart so inotropy and chronotropy are minimal. |
|
|
What is the main clinical use of vasopressin (V) receptors?
|
Causes very intense vasoconstriction.
|
|
|
What is the primary mediator of beta agonists?
|
To generate cyclic AMP which causes enhanced calcium influx and enhanced calcium release from the sarcoplasmic reticulum.
Inhibiting phosphodiesterase with drugs helps because it slows degradation of C-AMP. |
|
|
How do we increase preload?
|
Fluids
α-agonists in low doses |
|
|
How do we decrease afterload?
|
ACE inhibitors
Nitroprusside PDE inhibitors |
|
|
How do we increase contractility?
|
β-adrenergic agents
PDE inhibitors |
|
|
How do we increase vascular resistance?
|
α-adrenergic agents
|
|
|
What are the dosing ranges of epinephrine?
|
β-effects (Inotropy and chronotropy) predominate up to 0.1 μg/kg/min ~(5-7 μg/min)
α-effects at ~0.1 μg/kg/min Very non-specific beta agonist. Myocardial O2 demand is a real problem. |
|
|
What is Dobutamine (Dobutrex)?
|
Inotrope with Vasodilating effects.
+chronotrope Myocardial O2 demand may be less of problem than Epi. Typically used in acute MI and mild cardiogenic shock. |
|
|
What is the main effect of norepinephrine (Levophed)?
|
Mostly an alpha pressor
Some beta effects. Mostly used in Sepsis. (ie. Distributive shock with good CO) |
|
|
Phenylephrine (Neosynephrine)?
|
“Pure” weak α-agonist
Essentially no β-effects Vasoconstriction & increased SVR with no support of cardiac function. Mild Sepsis, Hypotension with good CO. |
|
|
What is the unique characteristic of dopamine (intropin)?
|
Dose-Dependent Pharmacology.
DA stimulation 0.5-3 μg/kg/min (renal dose dopamine) β-effects 2-10 μg/kg/min α-effects 7-20 μg/kg/min |
|
|
What is vasopressin (Pitressin)?
|
Causes Intense vasoconstriction through V-receptors with no α- or β- stimulation.
Predilection for splanchnic vessels can cause gut ischemia. Used in ACLS, Severe shock, CABG (on ACEi). |
|
|
What are the phosphodiesterase inhibitors milrinone and amrinone?
|
Vasodilators & Inotropes
Augments β-adrenergic stimulation. Used in CABG (Vasoconstricted with low CO) |
|
|
The most common cause of occlusive renal artery disease is...
|
Atherosclerosis.
|
|
|
How does renal artery stenosis cause problems?
|
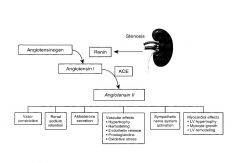
Once renal blood flow is less than 80%, the parenchyma receives less blood flow and causes it to release renin.
All problems stem from Renin causing increased conversion of angiotensin to angiotensin 1. |
|
|
What is the effect of unilateral renal stenosis?
|
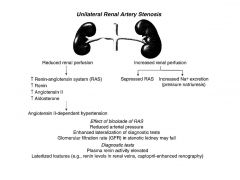
One kidney compensates but there is:
Reduced arterial pressure. Elevated plasma renin activity. Enhanced lateralization of diagnostic tests. GFR in stenotic kidney may fall. |
|
|
What is the effect of bilateral renal stenosis?
|
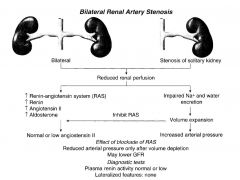
Reduced arterial pressure only after volume depletion.
May lower GFR. Plasma renin activity is normal or low. |
|
|
What are the clinical implications of renovascular hypertension?
|
Paroxysmal hypertension.
Treatment-resistant hypertension. Hypertensive nephropathy. Renal failure. Flash pulmonary edema. Accelerated cardiovascular disease. |
|
|
Among hypertensives: patients that are older, have higher diastolic pressure, and higher serum creatinine are more likely to have ...
|
Renovascular hypertension
|
|
|
What are the lesions responsible for adult renovascular hypertension?
|
Atherosclerosis (75%)
Fibromuscular Dysplasia (20%) Miscellaneous Lesions (5%) |
|
|
What are the causes of fibromuscular dysplasia of the renal arteries?
|
Medial Fibroplasia (85%)
String of Beads - Women (renal arteries, carotids,iliac) Intimal Fibroplasia (5%) Focal - Children Perimedial (Subadventitial) Dysplasia (10%) Progressive |
|
|
How does Isotopic captopril renography work?
(not the most important test to order but conceptually important) |
Measures filtered radioactive tracer before and after ACE inhibitor.
Angiotensin II maintains GFR in kidney with renal artery stenosis via increased vasoconstriction in the efferent arteriole. ACE inhibitor> reduced synthesis of Angiotensin II, diminished efferent arteriole vasoconstriction and thus decreased GFR. Therefore less uptake of filtered radioactive tracer after ACE inhibitor. If they have renal stenosis uptake will be markedly reduced. |
|
|
Statistically where do embolic phenomena from the heart go in the gut?
|
Superior mesenteric artery
(Feeds the majority of the small bowel) |
|
|
What are the collateral systems in the gut?
|
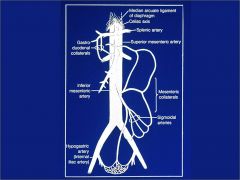
Celiac and SMA: Gastroduodenal collaterals
SMA and IMA: Mesenteric collaterals. IMA also connects with hypogastric (internal iliac artery) |
|
|
What is the relative incidence of the various visceral ischemic syndromes?
|
Mesenteric Artery Occlusion 75%
(Embolus 35%, Thrombus 65%) Mesenteric Vein Occlusion 15% Non-Obstructive Mesenteric Ischemia 10% |
|
|
What are the risk factors for visceral ischemic syndromes?
|
Any unexplained, severe abdominal pain
Age greater than 60 years Arrhythmia – atrial fibrillation. Congestive heart failure. Digitalis therapy (uncompensated CHF) Recent myocardial infarction Previous arterial emboli Hypercoagulable state Hypovolemia |
|
|
What should lead me towards the diagnosis of an embolic visceral ischemic syndrome?
|
Sudden onset severe abdominal pain (periumbilical)
Paucity of physical findings Bowel evacuation. Cardiac embolic risk such as atrial fibrillation. |
|
|
What should lead me towards the diagnosis of a thrombotic visceral ischemic syndrome?
|
Insidious abdominal pain
Systemic toxicity Physical findings Underlying chronic visceral ischemia |
|
|
What should lead me towards the diagnosis of a mesenteric vein occlusion visceral ischemic syndrome?
|
Insidious, diffuse abdominal pain.
Vague prodromal period. Nausea, vomiting, distention. Hypovolemic, cardiovascular collapse. |
|
|
What are the key symptoms of CHRONIC visceral ischemia?
|
Post-prandial abdominal pain
Food avoidance Weight loss |
|
|
How do people typically die of visceral ischemic syndromes?
|
Remote Organ Dysfunction - SIRS
Causes pulmonary endothelial cell Injury, Hepatocellular dysfunction and Renal insufficiency. |
|
|
How does the superficial system of veins drain into the deep system of veins in the leg?
|
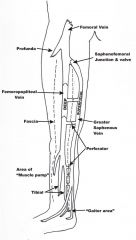
Direct connections at the saphenofemoral junction, saphenopopliteal junction and by perforators.
|
|
|
What affects the hydrostatic pressure in veins?
|
Static pressure of fluid column.
Transmitted right atrial pressure. Overall volume status. Thoracoabdominal pressure. |
|
|
What are varicose veins?
|
Genetic component – thought autosomal dominant with variable penetrance.
Valve Incompetence at: Saphenofemoral junction Saphenopopliteal junction Perforators Pain, aching sensation, worse as day proceeds, better in the morning. Compression stockings Elevation Intervention focuses on eliminating source of reflux. May need to remove varicosities. |
|
|
What are telangectasias? (spider veins)
|
Same process as varicose veins on a smaller scale.
Symptoms– cosmetic, sometimes pain at the site. Treatment – exclude larger vein reflux, injection sclerotherapy, laser. |
|
|
What skin changes are associated with venous stasis?
|
Lipodermatosclerosis
Pigmentation – hemosiderin deposition Eczema Ulceration – often at site of perforating vein. |
|
|
How do we manage venous stasis ulcerations?
|
Aggressive Compression.
Elevation. Antibiotics and debridement when infected or necrotic. Skin grafts. |
|
|
What is the CEAP classification of venous stasis disorders?
|
Clinical Signs, Etiology, Anatomy, Pathophysiologic dysfunction.
Allows standardized communication of venous disorders. |
|
|
What is virchows triad?
|
Stasis
Vessel wall injury Hypercoagulability |
|
|
What are the hypercoagulability risk factors for DVT?
|
Inherited thrombophilia
Malignancy Pregnancy Surgery Trauma |
|
|
What are some stasis risks for DVT?
|
Immobility.
Valvular dysfunction or venous obstruction. Pregnancy- pelvic vein compression. |
|
|
What sort of DVT risk does surgery pose?
|
Risk varies with type and length of operation.
Highest with hip/knee/pelvic procedures (~50-60%). Intermediate for neurosurgical and oncologic procedures. May be due in part to increased procoagulant activity Over 50% develop !!in the OR!! |
|
|
What is Homans sign?
|
Poor sensitivity and specificity indication of DVT.
Calf pain with dorsiflexion of the foot. |
|
|
What lab diagnostic test is useful for DVT?
|
D-dimers.
Venous Duplex in the vascular lab has very high sensitivity and specificity too. |
|
|
What is postphlebitic syndrome?
|
Symptomatic chronic venous insufficiency after deep venous thrombosis which occurs in ~28% of post-DVT patients.
|
|
|
Morbidity from DVT occurs due to...
|
Venous insufficiency.
Mortality is primarily due to pulmonary embolisms. |
|
|
Where do pulmonary embolisms come from?
|
>90% from legs.
Risk thought lower for calf vein DVT than more proximal DVT. |
|
|
What is a paradoxical embolism?
|
DVT that travels through a PFO (ASD) to the systemic circulation.
|
|
|
What means do we have of trying to prevent DVT?
|
Mechanical – compression stockings, intermittent compression devices, mobilization
Pharmacologic – low dose anticoagulants. |
|
|
Why is trauma a high risk for DVT?
|
Possibly due to depletion of coagulation inhibitors or upregulation of fibrinopeptides.
Increased even more with LE or pelvic fractures, spinal cord injuries, and major venous injuries. Frequently bilateral/ multifocal |
|
|
What is the usual treatment for DVT?
|
Anticoagulation.
Graduated compression stockings. Thrombolysis of clot in rare cases. |
|
|
What is Phlegmasia Cerulea Dolens?
|
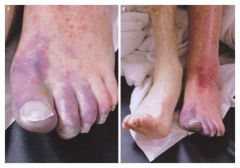
Limb threatening extensive venous thrombosis.
Often associated with cancer (20-40% of cases) Surgical/interventional emergency |
|
|
Some lymphatics have smooth muscle contractions but for the most part flow is driven by....
|
Variations in intrathoracic and intra-abdominal pressures.
|
|
|
What is the difference between lymphedema and edema?
|
Lymphedema = protein-rich interstitial volume overload, secondary to lymph drainage failure in the face of normal capillary filtration.
Edema = increased interstitial fluid volume that is enough to produce clinical, palpable swelling (more generic term) |
|
|
Primary lymphedema...
|
A congenital problem.
Secondary lymphedema is acquired. |
|
|
What is the most common worldwide cause of lymphedema?
|
Wuscheria bancrofti (filariasis)
In the U.S, the most common cause is malignancy and associated treatments. |
|
|
What are some primary lymphedemas?
|
Congenital (<1 year)
Familial type = Milroy’s Disease (VEGFR-3 mutation) Lymphedema praecox (age 1-35) Most common Usually unilateral, adolescent women. Hypoplastic lymphatics Lymphedema tarda (age >35) Congenitally “weakened” lymphatics, event triggers onset. |
|
|
What are the common signs and symptoms of lymphedema?
|
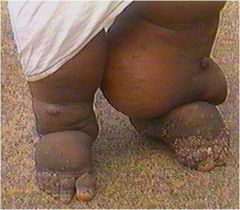
Painless swelling initially dorsum of foot.
Eventual proximal involvement. Subcutaneous fibrosis. Skin becomes hyperkeratotic, papillomatous or verucous Recurrent infections. |
|
|
How do we treat lymphedema?
|
No cure.
Elevation has little effect. Exercise. Compression garments/devices. Manual lymph drainage. Skin care practices. Surgery in rare cases. !Diuretics will not help! |
|
|
What is the principal modifiable risk factor for stroke?
|
Hypertension
Atrial fib and carotid stenosis are also risk factors. |
|
|
What percentage of strokes are ischemic?
|
80%
20% are hemorragic. |
|
|
Anterior strokes typically originate from...
|
The carotids.
The vast majority of arterial related strokes occur secondary to embolization. |
|
|
Speech issues are usually related to...
|
left sided strokes.
|
|
|
What is the most common cardiac source of embolisms?
|
Left atrium during A-fib.
|
|
|
The risk for embolic liberation of a carotid bifurcation plaque is related to....
|
The bulk of the plaque which is in turn related to the degree of occlusion (stenosis).
The stability of its luminal surface which is related to intraplaque hemorrhage and plaque composition. |
|
|
What are the greatest risks for atherosclerotic carotid plaques?
|
Advanced age
Cigarette smoking Not Hypertension. |
|
|
What is most common mode of presentation of carotid occlusion?
|
Asymptomatic
|
|
|
What are neurological symptoms of carotid derived stroke?
|
TIA
Amaurosis fugax Completed Stroke Vertebrobasilar symptoms* |
|
|
What is a TIA?
|
Unilateral symptoms with or without aphasia (left hemisphere) lasting less than 24 hours.
|
|
|
What is Amaurosis fugax?
|
Temporary ipsilateral monocular visual loss due to embolization of the temporal artery or its branches.
|
|
|
What is the gold standard for assessing carotid stenosis?
|
Carotid duplex
|
|
|
The best available natural history data on carotid disease comes from ....
|
Asymptomatic Carotid Atherosclerosis Study (or ACAS) demonstrated a five year risk of ipsilateral hemispheric stroke of 11% for lesions in excess of 60%. This equates to about a 2% per year risk.
The NASCET study which assesed symptomatic patients suggested 2 year stroke risk ~15-30% (50% or greater stenosis). The risk of strokes in either group parallelled lesion severity. |
|
|
How do we decide whether to treat patients with carotid disease medically or surgically?
|
Symptomatic patients < 50% diameter reducing stenosis and asymptomatic patients <60% stenosis are usually treated medically.
We recommend carotid endarterectomy plus optimal medical therapy for symptomatic patients with 50-99% carotid stenosis or asymptomatic patients with 60-99% stenosis and low perioperative risk. |
|
|
What are considered vetebrobasilar symptoms?
|
Dizziness
Vertigo Diplopia Blurred vision Ataxia Drop attacks Bilateral sensory disturbances These are typically due to low flow states rather than embolisms. |
|
|
What sort of flow limiting lesions cause vetebrobasilar symptoms?
|
almost always multiple vessels
(3 or more) Innominate artery Subclavian arteries Vertebral arteries (Retrograde flow) Carotid arteries -Systemic hypotension Bradyarrhythmias Tachyarrhythmias Autonomic dysfunction Anatomic compression |
|
|
What test is important if we suspect vertebrobasilar disease?
|
Transcranial doppler to assess intracranial flow.
In contrast to carotid disease, this often requires advancing to angio to fully asess. |
|
|
Known vertebrobasilar ischemia must...
|
Must be treated through bypass, endarterectomy, angioplasty and stenting depending on the lesion type and patient fitness.
|
|
|
What is Giant Cell Arteritis (temporal arteritis)?
|
Most common in older Caucasian women.
Temporal headaches Jaw claudication Polymyalgia rheumatica Amaurosis fugax Constitutional symptoms !Elevated sedimentation rate! Dx- temporal artery biopsy Tx-- corticosteroids |
|
|
What are the causes of carotid dissection?
|
Spontaneous:
Cocaine/methamphetamine use Associated Horner’s syndrome common. Treatment- anticoagulation Traumatic: Compression-extension injury Occurs high in the neck (C2) Treatment- anticoagulation if possible. Can lead to pseudoaneurysms. |
|
|
How do we define an aneurysm?
|
Focal dilatation of an artery involving an increase in diameter of at least 50% as compared the expected normal diameter.
True aneurysms- Have all the vessel wall layers. False aneurysms- Have none of the vessel wall layers. |
|
|
What are the most common aneurysms?
|
White males
Atherosclerotic Infrarenal Enlarge and rupture Greater than 60% of ruptured aneurysm patients never make it to the hospital. |
|
|
How does the matrix of collagen and elastin vary down the aorta?
|
Decreasing matrix concentrations from proximal to distal aorta.
58% decrease in elastin between supra and infrarenal aorta. (absent vaso-vasorum) |
|
|
What are some of the more exotic explanantions for the epidemiology of AAA?
|
Elastin is not synthesized in adult aorta (T-1/2 40 to 70 years) which explains age bias.
Genetics probably has to do with up-regulation of matrix metalloproteinases (MMPs). Susceptibility alleles for AAA involving the DRB1 major histocompatibility locus. Possible chlamydial pneumoniae infection cause.(some suggest give doxycycline). Aortic aneurysm antigenic protein (AAAP-40) shares AA sequence with treponema and CMV. Possible autoimmune reaction through molecular mimicry. |
|
|
What are the hazards with AAA?
|
Emboli
Thrombosis Infection Coagulopathy Fistulae !!Rupture!! |
|
|
How do we diagnose AAA?
|
Most are asymptomatic.
Occasionally patients describe abdominal pulsation or even palpate pulsatile mass. Abdominal or back pain (left flank). Can radiate to groin. Most asymptomatic aneurysms are picked up on Ultrasound or CT done for other reasons. |
|
|
Is angiography an adequate screening tool for AAA?
|
No.
|
|
|
What is the Law of Laplace?
|
Tension = radius x pressure.
|
|
|
What is the best screening tool for AAA?
|
Ultrasound.
Generally, 6 month interval ultrasound warranted for AAA > 4 cm. |
|
|
How can we slow the rate of expansion of an aortic aneurysm?
|
Beta blockers
|
|
|
What is the accepted cutoff for surgical repair of AAA?
|
5.5 CM
SOME USE A SMALLER CUTOFF FOR WOMEN AND COPD PATIENTS. |
|
|
What are the important comorbidities of AAA?
|
Coronary Artery Disease
Pulmonary Disease Renal Failure |
|
|
Several studies regarding smaller AAA (< 5 cm) have demonstrated...
|
Trend toward more rapid expansion above 5 cm
Trend toward rupture above 5 cm Recommendation for repair at 5 cm for younger, lower risk patients. Recommendation for repair at 6 cm for higher risk patients. Lower intervention rates at later age of detection. |
|
|
Fever of unknown origin or GI bleed with previous aortic grafting is...
|
Aortoenteric fistula or infected aortic graft until proven otherwise.
|
|
|
Who is a good candidate for aortic endograft?
|
Patients with an AAA warranting surgical repair that can tolerate a surgical procedure, can await/tolerate extensive imaging studies, are generally poor candidates for open repair and are committed to long-term follow-up.
|
|
|
What is the mortality of rupturing a AAA?
|
>90%
|
|
|
What is aortic dissection?
|
Separation of the aortic wall layers by extra luminal blood that usually enters the aortic wall through an intimal tear.
Relatively rare. BP must be controlled. Without treatment most will die within 2 weeks. |
|
|
What diseases predispose to aortic dissection?
|
Marfan, Coarctation, Ehlers-Danlos.
Cocaine use. Rebound from abrupt discontinuation of B-Blockers. Pregnancy. (More than 50% of dissection in women <40) |
|
|
Marfan syndrome is an autosomal dominant trait with variable penetrance. It is a defect in...
|
fibrillin-1
pectus excavatum, arachnodactyly, eye problems etc. |
|
|
What is the debakey classification of aortic dissection?
|
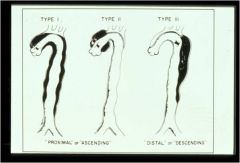
Type 1: whole arch
Type 2: ascending aorta Type 3: descending aorta Ascending aortic dissection is a true surgical emergency. 1%-3% for every untreated hour. May require replacement of ascending aorta, aortic valve, and CABG. Type 3 is the one that can sometimes be treated medically. |
|
|
How does an aortic dissection present?
|
Sharp, ripping, tearing pain (95%)
Abruptness is most specific characteristic. Usually does not radiate to neck, shoulder, or arm as in acute coronary syndrome. Location may reflect distribution of dissection. Typically hypertensive. Widened mediastinum. Acute aortic regurgitation (diastolic murmur) Shock: Pericardial tamponade Coronary compression MI Rupture Obstruction of branch arteries: Stroke / Spinal cord ischemia. Renal failure. Pulse Deficit. |
|
|
What else is on the differential with aortic dissection?
|
Acute MI
Thoracoabdominal Aneurysm Pericarditis Pulmonary Embolus Mediastinal Tumors Plueritis Acute Cholecystitis |
|
|
What imaging techniques can be used to diagnose aortic dissection?
|
CT, MRI, TEE, Angiography.
CT scan most common but center dependent. |
|
|
How do we treat dissection medically?
|
Aggressive blood pressure control with B-Blockers and nitroprusside.
A-line, ICU, CV access. Monitor for signs of progression or end-organ ischemia. Long-term aggressive blood pressure control and risk factor modification. |
|
|
What are the surgical indications for chronic descending aortic dissection?
|
Aneurysmal degeneration > 60 mm.
Chronic ischemia secondary to stenosis. |
|
|
What is a unique epidemiological charcteristic of lower extremity aneurysms?
|
Profound male predominance (30:1)
Largely Atherosclerotic/degenerative Popliteal most common followed by femoral. (femoral most common if include false aneurysms) !!Strongly Associated with Atherosclerotic aneurysms elsewhere!! (70% of patients with this will have aortic aneurysm) |
|
|
The danger with peripheral aneurysms is that...
|
More likely to embolize or thrombose than to rupture.
|
|
|
What are visceral aneurysms?
|
Splenic artery most common.
Biggest concern is rupture. Risk of rupture is increased with pregnancy. Tx if > 2.5-3.0 cm. especially if pt. pregnant. |
|
|
What is the most common site of mycotic aneurysms?
|
Femoral artery.
|
|
|
In most of the population, acute valve dysfunction and electrical conduction problems are due to an occlusion in the ...
|
Right coronary circulation.
|
|
|
What is MVO2?
|
myocardial oxygen consumption .
|
|
|
Coronary venous outflow is increased and peaks ....
|
During systole.
Coronary perfusion happens during diastole though which favors subendocardial vessels during diastole. |
|
|
What factors influence MVO2?
|
Heart Rate
Systolic pressure (or myocardial wall stress) Left ventricular contractility |
|
|
Are there collateral vessels in the normal human heart?
|
No.
Preexisting small vessels undergo proliferative changes of endothelium & smooth muscle in response to ischemia. May happen in as little as 3wks – 6 months. Vascular Endothelial Growth Factor (VEGF) is under investigation because of genetic link. |
|
|
Coronary flow is inversely proportional to...
|
Coronary vascular resistance.
|
|
|
What percentage of O2 does the myocardium extract from coronary blood flow?
|
75%
This is compared to the 25% extraction of most tissues. |
|
|
Why is the left coronary more affected by shortening of the diastolic phase than the right?
|
The mechanical compression of the right ventricle muscle is not as profound as that of the left ventricle during systole.
|
|
|
Myocardial pressure is greater at the endocardium than the epicardium so an excessively shortened diastole means change in the endocardium/epicardium perfusion ratio. Why is this not a problem under normal conditions?
|
Greater blood flow to the Endocardium in diastole compensates for greater blood flow to the Epicardium in systole.
Net result is about equal flow to epicardium & endocardium over the entire cardiac cycle. Ischemia & cell death usually start in the Endocardium & progress towards the Epicardium particularly in tachycardic states. |
|
|
What does increased sympathetic tone do to coronary blood flow?
|
Overall increased coronary blood flow.
BUT So does increased parasympathetic tone. |
|
|
What endothelial factors control coronary blood flow?
|
Prostacyclin (PGI-2) –relaxation via cAMP
Nitric oxide (EDRF-NO) – relaxation by ACh released NO, (cAMP mediated mechanism) Endothelin – potent vasoconstrictor assoc w/ injured endothelium Disease states alter the actions and release of NO, PGI2, and Endothelin. |
|
|
Basal MVO2 is about...
|
8-10 ml/min/100g tissue
|
|
|
How do we calculate arterial O2 content?
|
CaO2 =
1.38* Hgb * Sat+.003(PaO2) |
|
|
How is most of the myocardial oxygen used?
|
Pressure work 64% of total
Basal requirements 20% Volume work 15% of total Electrical activity 1% of total This means pressure overload has a bigger effect than a volume overload on ischemia. |
|
|
!! If we double HR, we increase MVO2 by...
|
Double.
1:1 |
|
|
!! If we double contractility, we increase MVO2 by...
|
90%
|
|
|
!! If we double the aortic pressure we increase the MVO2 by....
|
Double
1:1 |
|
|
If we double wall stress, we increase MVO2 by...
|
50%
2:1 |
|
|
If we double volume work, we increase MVO2 by...
|
8%
|
|
|
For one cardiac cycle, work is equal to...
|
W= Mean aortic pressure*SV
|
|
|
Coronary blood flow is distributed to chambers based on workload. Who gets the most in descending order?
|
LV>RV>LA>RA
|
|
|
Adenosine is formed by the myocardium when demand for O2 is greater than the supply immediately available. What does it do?
|
It binds adenosine receptors and causes vasodilation ie. increased coronary flow.
|
|
|
Coronary autoregulation refers to the ability of the heart to maintain constant coronary flow over a wide range of perfusion pressures. What are the bottom limits of this ability to autoregulate?
|
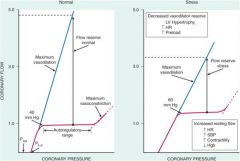
Mean pressure 40 mmHg
Diastolic pressure 30 mmHg |
|
|
What metabolic factors adversely affect coronary blood flow?
|
Decreased PO2
Acidosis |
|
|
What do hyperkalemic states adversely affect?
|
Myocardial contractility.
|
|
|
Under normal circumstances what substrates does the myocardium use for energy?
|
40% of MVO2 is due to oxidation of carbohydrates
60% is from fatty acids However, the heart uses whatever substrate is in highest concentration in the blood.Glucose and lactate are utilized at a similar rate. Ketone bodies can also be used. Pyruvate uptake is very low though. The hypoxic heart produces lactate & can utilize lactate as a substrate. |
|
|
In a normal heart, coronary flow can increase by about...
|
500-600%
|
|
|
What are the underlying goals of the treatment of ischemic heart disease?
|
Reduce CAD risk factors
Improve Supply & CBF Decrease Demand |
|
|
What are the typical ECG changes of an anterior MI?
|
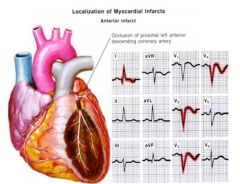
|
|
|
How do we improve oxygen supply to the myocardium?
|
Decrease HR:
Beta blockers (BBs) Calcium Channel Blockers (CCBs) Restore DBP: Intra-aortic balloon pump. Lower LVEDP: Nitrates CCB’s ACE inhibitors Angiotensin receptor blockers (ARB’s) Lower CVR: Revascularize Prevent thrombosis Antiplatelet agents Limit/reverse atherosclerosis Lipid lowering agents Dilate/relax coronary arteries with Nitrates (NTG, nitroprusside) and CCB’s Increase oxygen content: 1.38 x Hgb x O2Sat + 0.003 x PaO2 |
|
|
What do dihydropyridine CCB's like verapamil and diltiazem do?
|
Decrease heart rate.
Non-dihydropridine CCB's lower LVEDP and are a little more vasodilatory in the coronary circulation. |
|
|
What is a typical coronary venous oxygen saturation?
|
About 30%
|
|
|
If CBF, Substrate, or oxygen (MVO2) demand exceeds supply, ischemia may be either...
|
Regional or global
|
|
|
What is the standard paper speed for an EKG?
|
25mm/s
|
|
|
The gold standard for visualizing coronary arteries is...
|
Coronary angiography
|
|
|
What are the three acute coronary syndromes?
|
Unstable Angina
Non ST-Elevation Myocardial Infarction (NSTEMI); (Non Q Wave Myocardial Infarction) ST-Elevation Myocardial Infarction (STEMI); (Q Wave Myocardial Infarction) |
|
|
In a small subset of AMI there is no plaque rupture but erosion of the endothelium exposes plaque contents to thrombogenic forces in the blood. Who is this most common in?
|
Women
|
|
|
A small subset of MIs (less than 10%) occur in the absence of atherosclerotic plaque thrombosis stimulated by disruption or erosion. What are potential mechanisms of this?
|
Vasospasm
(cocaine users) Emboli (left sided cardiac thrombus, vegetation, paradoxical embolus) Unexplained secondary event. |
|
|
What are the charcteristics of an unstable plaque?
|
High lipid content and a thin fibrous cap.
|
|
|
What are Bivalirudin and Argatroban?
|
Direct thrombin inhibitors.
|
|
|
What is the main biochemical consequence of ischemia?
|
Anaerobic glycolysis within seconds.
Inadequate ATP and creatine phosphate as well as accumulation of noxious breakdown products like lactic acid. Without reperfusion - hypoxia and decreased removal of noxious metabolites (potassium, calcium, amphiphilic lipids, and oxygen-centered free radicals) leads to impaired ventricular function and predisposition to lethal arrhythmias. |
|
|
Where does ischemia usually start in the heart?
|
Subendocardium.
Progresses towards the epicardium. |
|
|
What is the effect of transient hypoxia and accumulation of metabolites on myocardial function?
|
Diminished diastolic relaxation.
Abnormal systolic contraction, (abnormal wall motion, and diminished stroke volume) |
|
|
What determines the size of a myocardial infarction?
|
Size of the vascular bed supplied by the obstructed vessel.
Duration of coronary occlusion. Metabolic/oxygen needs of myocardium at risk. Extent of collateral vessels. Alterations in blood pressure, heart rate, and rhythm. |
|
|
What is the diagnostic definition of an MI?
|
Detection of rise and/or fall of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit (URL) together with evidence of myocardial ischemia with at least one of the following:
• Symptoms of ischemia • ECG changes indicative of new ischemia (new ST-T changes or new left bundle branch block [LBBB]) • Development of pathological Q waves in the ECG • Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality. |
|
|
What are the diagnostic criteria for prior MI?
|
Development of new pathological Q waves with or without symptoms
Imaging evidence of a region of loss of viable myocardium that is thinned and fails to contract, in the absence of a non-ischemic cause Pathological findings of a healed or healing myocardial infarction |
|
|
What are the commonly used enzyme markers for MI?
|
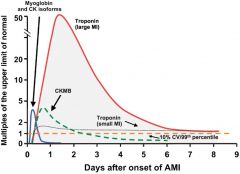
Creatine Kinase-MB- isoenzyme (CK-MB)
Troponin I and Troponin T (more specific) Aspartate serum transaminase(AST), Lactate Dehydrogenase(LDH1/LDH2 ratio) and myoglobin will also rise with acute myocardial infarction. |
|
|
What is the expected pattern for Creatine Kinase(CK) and MB isoenzyme in the setting of acute MI?
|
MB/total CK > 4%;
Initial rise within 4-6 hours, peaks within 16-24 hours, and returns to baseline within 3-4 days. |
|
|
What is the expected pattern for cardiac-specific troponins in the setting of acute MI?
|
T or I; rise within 4-6 hours; peak within 24 hours, remain elevated up to 7-14 days.
|
|
|
What pattern do we expect to see with lactate dehydrogenase in the setting of acute MI?
|
LDH1/LDH2 > 0.50;
Peak late and are elevated for several days. |
|
|
What trend do we expect for Aspartate Serum Transaminase (AST) in the setting of acute MI?
|
Intermediate time elevation.
(12-24 hours) |
|
|
The problem with myoglobin as an enzyme marker for MI is that it...
|
Is not specific for MI.
|
|
|
Tropnin levels correlate with ....
|
MI mortality.
|
|
|
What are the ECG findings of acute MI?
|
ST depression or deep T wave inversions in a consistent anatomical distribution suggest ischemia.
Hyperacute(Peaked) T waves (earliest signs of injury) ST elevation in a consistent anatomical distribution suggests ongoing myocardial injury. Diagnostic Q waves in a consistent anatomical distribution suggest prior myocardial infarct. |
|
|
What is the significance of ST Elevation in acute MI?
|
ST elevation in a consistent anatomical distribution suggests ongoing myocardial injury.
ST-Elevation MI (Q Wave MI) occurs when occlusive thrombi persist resulting in myocardial necrosis. Non ST-Elevation MI (Non Q Wave MI) results from incomplete or spontaneously recanalized thrombotic occlusions after ischemia that is persistent enough to elicit necrosis. In general patients with ST-elevation need fibrinolytic therapy while those with ST-depression or T-wave inversion with or without positive cardiac enzymes need potent antiplatelet therapy. (GP IIb-IIIa inhibitors ) |
|
|
What does non-ST elevation MI (a cousin of unstable angina but with elevated enzymes) tell us?
|
Total occlusion of culprit vessel much less likely vs. ST elevation MI.
More likely to have multivessel CAD. More likely to have prior MI's More likely to have history of diabetes, hypertension, heart failure, and peripheral vascular disease. Less likely to be smokers or have dyslipidemia. Elderly patients more likely to have non-ST elevation MI's. |
|
|
Changes in which ECG leads correlate with which vessels?
|
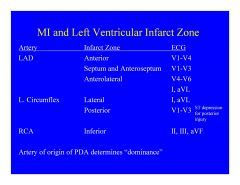
II, III and aVF:
inferior MI RCA V1-V4: Anterior MI LAD I and AVL: Lateral MI L.circumflex or Anterolateral MI LAD (With V4-V6) V1-V3: Septum and anteroseptum MI LAD (with ST elevation) or Posterior MI L.Circumflex (With ST depression) |
|
|
How do we know the dominance of a coronary circulation?
|
Artery of origin of PDA determines “dominance”.
|
|
|
What determines the prognosis of MI?
|
Early prognosis is determined by presence of arrhythmias, sudden cardiac death, and development of complications.
Late prognosis is determined by left ventricular function. |
|
|
What are clear indications of high risk MI patients on admission?
|
Heart rate > 100 bpm
BP < 100 mm Hg Pulmonary edema(rales greater than one half way up) Shock |
|
|
What are the complications associated with acute MI?
|
Arrhythmias: heart block, bradycardia, or ventricular arrhythmias.
Congestive heart failure and pulmonary edema. Cardiogenic shock Pericarditis: early or late(Dressler’s Syndrome) Mechanical Complications: ventricular septal defect; papillary muscle rupture with development of severe mitral regurgitation; or rupture of the left ventricular free wall. Development of scar or ventricular aneurysm. |
|
|
What should we look out for with inferior wall MI?
|
RV ischemia occurs in up to half of all inferior infarcts
(only 10-15% show classic hemodynamic abnormalities) RV infarction accompanying inferior infarct=significantly higher mortality(25-30%) High priority candidate for reperfusion. |
|
|
What is a secondary AMI?
|
infarcts may occur secondary to insufficient perfusion relative to myocardial demand during certain pathologic conditions.
Anemia Hypoxia Acidemia Infection High catecholamine state. |
|
|
What is the algorithm for managing non ST elevation MI?
|
|
|
|
What is the algorithm for managing ST elevation MI?
|
|
|
|
What factors favor an invasive strategy in non ST elevation acute coronary syndromes?
|
Recurrent angina or ischemia at rest or with low-level activities despite intensive medical therapy.
Elevated cardiac biomarkers. New or presumably new ST segment depression. Signs or symptoms of heart failure or new or worsening mitral regurgitation. Hemodynamic instability. Sustained ventricular tachycardia. PCI within 6 months. Prior CABG. High risk score. Reduced left ventricular function(EF < 40%). |
|
|
Which cardiac ion channels are passive?
|
Sodium
Calcium Potassium |
|
|
What do we mean when we say ion exchangers?
|
Ion exchangers are selective (for at least two ions) and passive.
Unlike ion channels they can reverse directions. Na/Ca exchanger in cardiac muscle. |
|
|
Ion pumps are selective, and actively transport ions across membranes against concentration or voltage gradients. Which ones exist in cardiac muscle?
|
Na+/K+ pump
Ca2+ exporter |
|
|
What is the resting potential membrane potential of a cardiac cell?
|
-90mV
Based on Nernst equation. |
|
|
What is the intracellular ion content of a cardiac cell in the resting state?
|
High K+
Low Na+ Low Ca2+ Resting potential is primarily due to Na+/K+ pump. K+ wants to rush out. Na+ wants to rush in. |
|
|
What is happening electrophysiologically when a cardiac myocyte is at rest?
|
Na+ channels are closed
Ca++ channels are closed Some K+ channels are closed Some K+ channels are open Cell is actively pumping out Na+ and Ca++ K+ is leaking out passively, leaving behind a negative intracellular charge (– 90 mV) |
|
|
What is the threshold potential of cardiac myocytes?
|
-70mV
|
|
|
What causes the ion channels to open in a cardiac myocyte?
|
Voltage.
They are said to be voltage gated channels. |
|
|
How is the depolarization of a cardiac myocyte different from a neuron?
|
It is much longer
|
|
|
What are the primary channels responsible for myocyte depolarization?
|
Voltage gated Na+ channels.
Activate rapidly, and inactivate rapidly. Inactivation happens so rapidly that it occurs before any of the other channels have even responded. Can be closed, open or inactivated. |
|
|
What is phase zero of the cardiac action potential?
|
Rapid depolarization due to fast sodium channel opening.
|
|
|
What is phase 1 of the cardiac action potential?
|
Transient outward current – mostly K+ efflux.
Small repolarization immediately following phase 0 (rapid depolarization). |
|
|
What is phase 2 of the cardiac action potential?
|
Plateau phase
All about calcium. Depolarization of the cell triggers Ca++ channels to open. Slower and sustained Ca++ influx prolongs depolarization, and triggers release of Ca++ from the Sarcoplasmic Reticulum which precipitates contraction through calcium activated calcium release. (excitation-contraction coupling) |
|
|
What is phase 3 of the cardiac action potential?
|
Rapid repolarization.
Ca++ channels close. Voltage-gated K+ channels remain open, repolarize cell. Na+ channels begin to revert to resting (closed) state. |
|
|
What is phase 4 of the cardiac action potential?
|
Resting state.
All Na+ channels closed etc. |
|
|
When does phase 4 of the cardiac action potential happen?
|
Diastole
(end of T wave to beginning of P wave) All other phases happen during systole. |
|
|
How do Na+ channel blocking drugs affect the cardiac action potential?
|
Slows upstroke of phase 0 so its not a line straight up anymore.
!!Widens the QRS complex on ECG!! |
|
|
How do K+ channel blocking drugs affect the cardiac action potential?
|
They prolong phase 3 rapid repolarizaton and therefore prolong the QT interval on ECG.
|
|
|
What is the absolute refractory period?
|
Beginning of phase 0 to end of phase 2.
All Na+ channels are inactivated – cannot reopen in any circumstance; must first get to resting (closed) state. All Ca++ channels are open Cell is completely inexcitable No stimulus of any strength is able to generate a new action potential. Effective refractory period is absolute refractory period plus a small portion of phase 3. |
|
|
What is the relative refractory period?
|
Phase 3.
Cell is beginning to repolarize. Cell has not yet returned to resting state. Most Na+ channels are still inactivated but some are moving towards resting (closed). Ca++ channels are mostly closed which means they can reopen. A strong stimulus can induce an action potential that will be lower amplitude and slower velocity than “normal” but the action potential will be strong enough to propagate normally to adjacent cells. |
|
|
What happens if a premature beat or pacing stimulus arrives during the effective refractory period?
|
No response
|
|
|
What hapens if a premature beat or pacing stimulus arrives after the Relative Refractory Period?
|
Normal action potential
|
|
|
What happens if a sufficiently strong premature beat or pacing stimulus arrives after the Effective Refractory Period but during the Relative Refractory Period?
|
Slower-onset action potential within the cells directly activated, but normal propagation to adjacent cells.
|
|
|
What is a pacemaker current?
|
Slow influx of positive ions into cells with automaticity (during the normally flat phase 4) that eventually reaches threshold and causes spontaneos depolarization.
This happens in cells of the conduction sytem or in myocytes that have been ischemically or metabolically injured. |
|
|
“What is the "resting” potential for Sinus and AV nodal cells and how is their pattern of depolarization different?
|
-60mv
Na+ channels are not active in Sinus or AV nodal cells so depolarization happens through calcium channels. Ca++ channels are slower than Na+ channels so: Phase 0 has a slower, more gradual rise. Phase 1 does not occur because it is a response to rapid Na+ “overshoot” in other cells. Phase 2 also doesn’t occur since the calcium channels are used up driving phase 1. Phase 3 still occurs normally because voltage-gated K+ channels are the same as in other cells. The pattern of depolarization in these cells is therefore almost sinusoidal. |
|
|
From an electrophysiological standpoint, why can the atria go faster than the ventricles?
|
They have a shorter effective refractory period.
Atrial Fibrillation (>350 bpm) Atrial Flutter (300 bpm) |
|
|
What makes the sinus node the dominant rhythm?
|
Under normal conditions, sinus node automatic rhythm is faster than any other location.
|
|
|
What makes AV nodal conduction slower than atrial, ventricular and His-Purkinje cells?
|
Phase 0 in the AV node is driven by slow Ca++ channels instead of fast Na+ channels.
Intrinsic rythm is 40-60bpm but can produce accelerated junctional rhythm (>60bpm) |
|
|
What is the refractory period of the His-purkinje system relative to atrial cells?
|
Much longer.
|
|
|
Why is impulse conducted fast in the His-purkinje system?
|
Greater density of Na+ channels than other cells.
Greater density of gap junctions than other cells. |
|
|
Which cells have a longer refractory period, atrial or ventricular myocytes?
|
Ventricular myocytes.
|
|
|
How does the autonomic nervous system influence AV nodal conduction?
|
Sympathetic stimulation increases conduction velocity and automaticity.(+chronotropy and dromotropy)
Parasympathetic stimulation decreases conduction velocity and automaticity. (-Chronotropy and dromotropy) Probably mediated mainly by indirect effects on Ca++ channel function. |
|
|
How do chronotropic drugs affect the conduction system in the heart?
|
Alter automaticity
(Usually SA node) |
|
|
How do dromotropic drugs affect the conduction system in the heart?
|
Alters the speed of propagation of an impulse.
(usually AV conduction) |
|
|
What is a physiologic AV block?
|
Long refractory periods in the presence of abnormally fast atrial rhythms protect the ventricles.
|
|
|
What are the normal automaticity rates of the conduction system?
|
Sinus Node:
60- 100 bpm (at rest) AV Node and His bundle: 40- 60 bpm Bundle Branches, Purkinje system: 30- 40 bpm |
|
|
Junctional escape rhythms originate from...
|
The AV junction
|
|
|
Ventricular escape rhythms originate from...
|
The Purkinje network.
|
|
|
What can we say about the reliability of the different escape or ectopic rhythms?
|
Ectopic atrial rhythm:
Usually pretty reliable AV Node +/- His bundle: Good, but a little less reliable. More easily suppressed than sinus or atrial rhythms. Purkinje network: Not reliable. Easily suppressed. |
|
|
What are the three primary mechanisms for tachyarrythmias?
|
Abnormal Automaticity. (ischemia or metabolic)
Triggered Activity. (afterdepolarizations) Reentry phenomena. |
|
|
What is an afterdepolarization?
|
Spontaneous depolarization occurs during or just after the previous action potential.
If the afterdepolarization reaches threshold it generates another action potential. Early afterdepolarizations interrupt phase 3 of the first AP and are usually caused by reactivation of Ca++ channels. They prolong the QT interval, they can be self-perpetuating and they can preciptate dangerous rhythms like torsades depointes. Delayed afterdepolarizations occur during phase 4. These mostly happen in the setting of intracellular Ca++ overload. Associated with Dig. Toxicity but other arrhythmias also have this mechanism. |
|
|
What is the most common mechanism of tachyarrythmias?
|
Re-entry phenomena
|
|
|
What conditions need to be met for a re-entry phenomenon to occur?
|
There need to be at least two pathways for electrical current.
Unidirectional block needs to be possible in at least one pathway. There needs to be sufficient difference in the speed of the pathways to get re-entry going. |
|
|
What is typical AV node re-entry?
|
Impulse goes down the slow pathway and back up the fast pathway.
Almost always due to a premature beat Premature beat comes in very soon after the preceding beat and the fast pathway is still refractory from the previous beat. The fast pathway therefore cannot be activated by the premature beat. The ERP of the cells (!!not the “speed” of the pathway!!) determines whether the unidirectional block will occur. |
|
|
What is “atypical” AV node reentry?
|
Prototype example Wolf-parkinson-white syndrome.
In WPW, the accesory pathway is the fast pathway and the AV node is the slow pathway. The reentrant loop uses the slow pathway (AV node) as the retrograde limb. Causes atrioventricular re-entrant tachycardia. |
|
|
What is the primary mode of V-Tach after an MI? (possibly after reperfusion)
|
Differences between damaged and healthy tissue cause a difference in conduction (slow and fast pathway) which allows for re-entry phenomena.
|
|
|
What is though to be the cause of Idiopathic Ventricular Tachycardia?
|
Re-entry in the ventricles.
|
|
|
What are the classes of antiarrhythmic drugs?
|
Class 1 –
Na+ channel blockers Further divided into subgroups 1A, 1B, 1C Class 2 – Beta blockers Class 3 – K+ channel blockers Class 4 – Ca++ channel blockers (CCB) Others – adenosine, digoxin |
|
|
Where are beta receptors found?
|
β-1 receptors in heart.
β-2 receptors in vascular smooth muscle and bronchial smooth muscle. |
|
|
What are beta blockers? (Class 2 antiarrythmics)
|
Only "antiarrrythmics that improve survival".
Don't actually affect the heart directly but reduce sympathetic stimulation. Slow automaticity in sinus node AND AV node by slowing phase 4 depolarization. -Chronotrope, -dromotrope,-inotrope Can induce bradycardia. Cardioselective examples: Atenolol Metoprolol Esmolol Non-selective beta blockers: Propranolol Labetalol – also α-block Carvedilol – also α-block Non-selectives can exacerbate asthma and Raynaud's. IV Labetalol useful in hypertensive emergencies because of alpha blockade. |
|
|
What two subclasses of CCB's (class 4 antiarrythmics) exist?
|
Dihydropyridine (DHP):
Nifedipine, Amlodipine, Felodipine, etc., etc. Nondihydropyridine (non-DHP): Diltiazem Verapamil |
|
|
Why do only diltiazem and verapamil (Non-DHP CCB's) have clinically relevant effects on cardiac action potential amongst the CCB's?
|
DHP-CCBs are such potent vasodilators that no one can take a high enough dose to actually affect the cardiac Ca++ channels.
|
|
|
How do diltiazem and verapamil work?
|
Block the L-type calcium channels in the Sinus and AV nodal cells. (!!AV node most!!)
Similar clinical effect to beta blockers. Negative chronotropy Negative dromotropy Negative inotropy (major problem unlike in beta blockers, relatively contraindicated in sytolic heart failure) Usually do not cause significant sinus bradycardia unless patient already has sinus node dysfunction. If patient does have sinus node dysfunction, the effect can be profound. Can “shut down” a junctional escape rhythm. Especially useful to slow ventricular response rate during atrial tachyarrhythmias. Can interrupt any reentrant loop that uses the AV node as part of the circuit ( eg. AV Node Reentry, AV Reentrant Tachycardia) Can cause peripheral edema. Various drug interactions often guide use. |
|
|
By what mechanism is Digoxin a positive inotrope?
|
Digoxin poisons the Na+-K+ pump.
Increases Na+ concentration inside cell. Na+-Ca++ exchanger keeps more Ca++ in cell to balance this. More Ca++ enters SR and therefore more Ca++ is available for contraction. |
|
|
How is digoxin an antiarrythmic?
|
Primary effect on cardiac rhythm is through augmentation of vagal tone.
Primarily affects Sinus and AV nodes. Like verapamil and diltiazem the AV node is the primary target. The sinus node is not usually affected, but can be profoundly supressed in patients with pre-existing sinus node dysfunction. Much less effective in slowing AV nodal conduction than verapamil and diltiazem but does not cause hypotension. Narrow therapeutic index. |
|
|
What are the charcteristics of Dig toxicity?
|
Nausea / vomiting, visual disturbances (seeing yellow-green halos around things).
High-grade AV block along with atrial tachycardias. Ventricular tachyarrhythmias due to delayed afterdepolarizations. Digoxin is cleared by the kidney, so must keep track of renal function. Severe toxicity may need digibind and dialysis. |
|
|
What are the half life and side effects of adenosine?
|
about 10 seconds
Can precipitate bronchospasm in asthmatics which may persist after adeonsine is cleared. Rarely can precipitate atrial fibrillation but typically not with usual doses (6mg-12mg). |
|
|
What is adenosine used for?
|
First line drug for terminating AV node involving re-entry.
Slows the sinus. No noticeable effect on ventricular cardiac cells. |
|
|
Which drugs are specifically targeted toward the cardiac action potential?
|
Class 1 and 3 antiarrythmics.
Have direct effects on atrial and ventricular cells. |
|
|
What do class 1 antiarrythmics do?
|
Na+ channel blockers.
Make fewer Na+ channels available for conduction. Cumulative effect is to slow conduction through the affected tissue. Raise the threshold somewhat for initiation of action potential. Slow the upstroke of phase 0. May also decrease slope of phase 4 in cells with abnormal automaticity. Slows or terminates automaticity but sinus node not usually affected unless already have sinus node dysfunction. Can resolve rentry issue but can also exacerbate them. This is the primary danger of – incessant VT. Patients with prior myocardial infarction should not take type 1 drugs unless they already have an ICD. |
|
|
What are the drugs classified as class 1 antiarrythmics?
|
Class 1A:
Quinidine Procainamide Disopyramide Prolong QRS and QT (Torsades). Also block K+ channels. Lots of side effects. Class 1B: Lidocaine Mexiletine Only work in ventricle. Preferentially target ischemic cells. Shorten QT. Class 1C: Flecainide Propafenone Most potent. Prolong QRS the most. No direct effect on QT. Negative inotropes (bad in heart failure). |
|
|
What are the main side effects of quinidine?
|
Major GI side effects (N/V/D)
“cinchonism” = tinnitus, confusion, visual disturbance, etc. Autoimmune thrombocytopenia Major interaction with digoxin (Raises digoxin level) |
|
|
What is unique about procainamide?
|
Only 1A drug used intravenously.
Breakdown product = NAPA (N-acetyl procainamide) active metabolite. Patients can be slow or fast acetylators but we check both Procainamide + NAPA for blood levels. Lupus-like syndrome in up to ⅓ of patients with Fever, rash, arthralgias, etc. Requires stopping the drug. |
|
|
What is unique about the class 1A antiarrythmic Disopyramide?
|
Strong anticholinergic effect:
Dry mouth, urinary retention, constipation, exacerbation of glaucoma etc, -inotrope unlike procainamide and quinidine. |
|
|
What is the major concern with class 1A antiarrythmic?
|
Class 1A drugs also block K+ channels to some extent.
They can prolong the QT interval. This can cause Torsades de pointes (aka polymorphic ventricular tachycardia). |
|
|
Lidocaine was once recommended prophylactically during AMI why?
|
Preferentially affects ischemic tissue and could be useful for ventricular arrythmias during AMI.
HOWEVER, Trial data proved this false – it increased mortality in AMI. |
|
|
Why is it important to remeber that lidocaine is cleared by the liver?
|
Liver disease (obviously).
Heart failure means less hepatic perfusion. Lidocaine also has serious CNS side effects at high levels, confusion and seizures. Levels should be monitored carefully for prolonged continuous infusion. |
|
|
What is mexilitene?
|
Oral only
Practically speaking, probably okay to consider this an oral form of lidocaine. Not very effective, but mixes well with certain other antiarrhythmics when needed. |
|
|
What class of drug is Phenytoin (dilantin) ?
|
technically a class 1B antiarrythmic but is almost never used for this purpose.
|
|
|
What are the class 1C antiarrythmics Flecainide and Propafenone?
|
The most potent Na+ channel blockers.
Markedly decrease upstroke of phase 0 “Clean” drugs – no effect on K+ channels. No direct effect on QT interval. Can cause bad arrhythmias, but not Torsades. Negative inotropes (contraindicated in systolic heart failure) Primarily used for atrial arrhythmias (AFib) Also can be used to “shut down” accessory pathways (i.e. Wolff-Parkinson-White syndrome. Flecianide side effects like fatigue and visual disturbance are common, but not usually severe enough to stop the drug if it’s working. Propafenone has beta blocking properties and can produce dizziness. How much depends on individual patient’s metabolism. |
|
|
What do we mean by the use dependance of Na+ channel blockers?
|
Na+ channel blocking drugs bind preferentially to open channels. The more the channels are open (being used), the greater the blockade
This means that Na+ channel blockers are even more effective at faster heart rates like a-fib (300-400bpm) and may even convert it back to sinus. |
|
|
What do mean when we say that K+ channel blockers (Class 3 antiarrythmics) have reverse use dependance?
|
K+ channel blockers have greater effect during slower heart rates.
This makes them less useful in terminating atrial fibrillation (when atrial rates are very fast) than sodium channel blockers. It also means that bad arrhythmias such as Torsades are more likely during slower heart rates if the QT is prolonged. “pause-dependent Torsades” = Polymorphic VT occurring after a sinus pause. |
|
|
Which drugs are the potassium channel blockers (Class 3 antiarrhythmics)?
|
Sotalol
Dofetilide and Ibutilide Amiodarone Dronedarone |
|
|
How does hypokalemia influnce the use of potassium channel blockers?
|
Effect of the drug is magnified.
|
|
|
What are some caveats to keep in mind when we use potassium channel blockers?
|
They all prolong the QT interval.
Torsades (polymorphic VT) is a risk with most (exception = amiodarone) Torsades is more likely when: Too much drug on board Renal or hepatic failure, depending on drug. Hypokalemia Bradycardia and especially sinus pauses. Despite all this, K+ channel blockers can be used in patients with prior AMI. |
|
|
What are some caveats specific to the Class 3 antiarrhythmic Sotalol?
|
Also has beta blocker activity.
Can be good or bad depending on patient. Cleared by the kidney. Can accumulate in renal failure and cause Torsades. QT must be monitored periodically. |
|
|
What are the class 3 antiarhythmics Dofetilide and Ibutilide?
|
Ibutilide = intravenous; Dofetilide = oral
Otherwise, basically same characteristics. Ibutilide is given IV to convert Afib. Torsades in up to 10% Can partially prevent by giving IV magnesium first. Dofetilide is cleared by liver and kidney. Renal clearance seems especially important. Dofetilide is relatively safe in heart failure. Not a negative inotrope QT must be monitored periodically |
|
|
What is amiodarone?
|
Class 3 antiarrhythmic.
Has properties of all four antiarrhythmic classes: Some Na+ channel blocking. Some β-blocking. A lot of K+ blocking. Some Ca++ channel blocking. Probably why it works for almost every arrhythmia. “safest antiarrhythmic" drug for your heart, and most dangerous one for every other part of your body. Has a LOT of iodine which contributes to noncardiac toxicities. Prolongs QT but very rarely causes Torsades (QT monitoring not necessary) Almost never causes ventricular arrhythmias but can’t be counted on to prevent them. Only ICDs have been shown to improve mortality in patients at risk for fatal arrhythmias. Irreversible pulmonary toxicity is most serious but can take up to ten years to develop. Not a drug for young people. Thyroid issues, liver injury photosensitivity and bluish skin with high doses also a problem. (Blue, blind and coughing up your liver and thyroid) |
|
|
What is Dronedarone?
|
New drug similar to amiodarone.
No iodine Less toxicities May not be as protected from Torsades. QT must be monitored but Torsades risk probably low. Interferes with creatinine secretion – raises serum creatinine level but GFR is actually not affected. |
|
|
Which antiarrhythmics are okay to give in the setting of systolic heart failure?
|

|
|
|
Which antiarrhythmics are okay to give in the setting of prior MI with myocardial scar but without systolic heart failure?
|

|
|
|
Which antiarhythmics are best for a healthy ventricle?
|
Everything is okay (hooray!)
Type 1 antiarrhythmics (especially 1C – flecainide and propafenone) may actually be safer than type 3 drugs in patients with a structurally normal ventricle (no scar, normal systolic function etc.) |
|
|
Leading cause of mortality in adult women?
|
CV disease
|
|
|
True or False:
Men have first MI ~10 years before women have their first MI. |
True
|
|
|
True or False:
Women are just as likely to have ST segment elevation MI as men, but clinicians are less likely to recognize it. |
False
Women are less likely to have ST segment elevation, and have higher prevalence of “silent MI”. |
|
|
Women often have "atypical" pattern for angina. What is this pattern?
|
neck/jaw pain, dyspnea, or fatigue
|
|
|
What is a goal LDL for those with CHD or high risk?
|
<70
|
|
|
What is a goal LDL for those with 2 or more risk factors for CHD?
|
<100
|
|
|
What is a goal LDL for those with 0-1 risk factors for CHD?
|
<160
|
|
|
What are some of the gender differences in risk factors for CHD?
|
Diabetes is a much stronger predictor of CHD risk in women than in men.
High Triglycerides carry greater risk in women. Physical inactivity more prevalent among women. Smoking may be stronger risk factor in middle-aged women. CRP levels are higher in women. (iffy) |
|
|
What key processes in the development of CHD does estrogen affect?
|
Lipid levels
Arterial vasodilation Clot formation/breakdown Some evidence to suggest that progesterone attenuates estrogen’s favorable effects. |
|
|
What effects does estrogen have on endothelium?
|
Stimulates NO synthesis
(Vasodilation Increases Plasma renin activity Inhibits ACE activity Protects LDL from oxidation Increases endothelial regrowth |
|
|
How does estrogen affect coagulation?
|
!!procoagulant!!
Increases Prothrombin Decreases antithrombin III Contributes to increased venous thromboembolic events. |
|
|
What is the result of injury to various blood vessel layers?
|
Endothelium:
Thrombi, thickening, & spasm leading to pain of ischemia & loss of function of organ. Media: Aneurysm; rupture leading to pulsatile mass, hypotension and pain. Adventitia: Weakening, false aneurysm with resulting lump(hematoma), pain. |
|
|
How do various size arteries respond to injury?
|
Large vessels:
Aneurysm , rupture. Medium arteries: Thicken and narrow (stenosis) or thrombose which may lead to tissue ischemia. Small arteries & arterioles: Arteriolar narrowing can cause hypertension. Capillaries: Petechial hemorrhage, microthrombi (DIC), narrowing in diabetes. |
|
|
In broad terms, what are the three types of athersclerotic lesions in vessels?
|
Fatty streak
(small, intracellular fat , "innocuous") Atheroma (raised, lipid center "dangerous") Fibrous plaque (intimal scar, "innocuous") |
|
|
What are the three major elements of an atheroma?
|
Necrotic center of extracellular lipid
Fibrous cap Proliferating cells (myofibroblasts) |
|
|
What are the possible complications of an atheroma?
|
Ulceration (of the plaque)
Thrombosis (often caused by ulceration) Medial damage, causing aneurysms. Hemorrhage into plaque. Calcification. |
|
|
What are the implications of arterial inflammation (arteritis)?
|
It can:
Weaken vessel walls causing aneurysm or rupture. Narrow the lumen causing ischemia. Damage the endothelium, resulting in thrombosis. Become complicated by arteriosclerosis (e.g., syphilitic aortitis with secondary atherosclerosis) |
|
|
What is Kawasaki disease?
|
AKA muco-cutaneous lymph node syndrome
Idiopathic Necrotizing Arteritis. in children (typically under 5) with skin and lymph node involvement as well as rash. Sometimes (~10%) includes autoimmune coronary arteritis and aneurysmal dilation of the coronaries. Can also cause unexpected late stage death due to cornary aneurysm. Presumably virally related but we dont know which virus. |
|
|
What is Takayasu arteritis?
|
An Idiopathic Necrotizing Giant CellArteritis.
Causes thickening of aortic arch. Blindness/CNS deficits(carotids) ↓pulses in upper extremities, ‘pulseless disease’. Women, 15- 45 years most commonly affected |
|
|
What is thromboangiitis obliterans (Buerger disease)?
|
An Idiopathic Necrotizing Arteritis involving pain and ischemia, legs and arms.
Relatively young men. Leads to gangrene of extremities. Genetic predisposition possible. Almost exclusively in smokers May improve with smoking cessation. |
|
|
What is phlebitis?
|
Acute inflammation of veins, usually with thrombosis.
Caused by bacterial, parasitic, physical, chemical, allergic injury. Examples: Pylephlebitis (portal vein) from abdominal infection (e.g., appendicitis) Dural sinuses in infections of ear and face. Pulmonary veins in pneumonia. Iliofemoral veins in puerperal sepsis. Hepato-veno-occlusive disease from chemotherapy. Migratory thrombophlebitis- "Trousseau syndrome"- Transient attacks, variable sites Associated with internal cancers (e.g., pancreatic CA). Idiopathic Thrombophlebitis (phlebothrombosis) |
|
|
What disease is associated with migratory thrombophlebitis?
|
Pancreatic cancer.
|
|
|
What is phlebothrombosis?
|
Venous thrombosis without inflammation. (no -itis)
Usually in deep veins of legs (DVT) or pelvic veins. Exact incidence uncertain, but high (autopsy patients, as high as 60%) Pain, tenderness, swelling, or may be asymptomatic. Up to ~50% can develop pulmonary embolisms. Clots usually form in relation to valve cusps (sites of maximum stasis) and propagate upward. |
|
|
What are varicose veins?
|
Abnormally dilated, tortuous veins.
Produced by prolonged increase in intraluminal pressure. Often in relation to injury or defect of the vein proximally. Different names at different sites. (Varciocele-testes, Hemmorhoids-perianal etc.) Do NOT cause pulmonary embolism. Too superficial to get into the deep veins. |
|
|
What is the mechanism of atherosclerotic aneurysms?
|
Atheromas cause medial destruction through ulcerated plaques.
|
|
|
What are the complications of athersclerotic aneurysms?
|
Rupture (retroperitoneal hemorrhage),
Stenosis of ureter by pressure or fibrosis, Occlusion of aortic branch (renal, mesenteric), Embolism (thrombo- or atheromatous) |
|
|
What is the mechanism of syphillitic aneurysm?
|
Tertiary syphilis involves vasa vasorum.
Causes vasculitis, and medial damage. Presentation varies but usually includes dyspnea, stridor, dysphagia, cough, pain congestive heart failure due to aortic insufficiency (AI). Tree barking on intima commonly seen post-mortem. |
|
|
What causes mycotic aneurysms?
|
"Mycotic" refers to any infection, not necessarily fungal.
Infection causes destruction of media. Can complicate to distal or bland infarcts. Typically one of the Sequelae of infective endocarditis or endarteritis. |
|
|
What is a dissecting hematoma of the aorta?(pseudoaneurysm)
|
AKA Aortic dissection
Age 40-60, males 6:1 95% have intimal tear in ascending aorta. 5% have no intimal tear (probably just small) If hemorrhage extends to aortic branches, can cause differences in pressure and pulse in arms or legs. Type A: Most common - involves ASCENDING AORTA & may extend distally Type B: Begins distal to subclavian artery, extends distally (3x better prognosis than Type A, because does not involve vessels to head) Type A can cause Hemopericardium from proximal rupture and aortic valve damage. Associated with hypertension, trauma and Marfan's syndrome. |
|
|
How do we calculate QTC?
(corrected QT interval) |
QT interval/(square root of R-R interval)
QTC is more important for following drug effects and is usually calculated by the machine. |
|
|
What is the primary etiology of valvular heart disease?
|
Rheumatic heart disease has been displaced by degenerative disorders as the primary etiology.
|
|
|
Coarctation has an association with...
|
A bicuspid aortic valve.
|
|
|
What valve disease are dialysis patients at high risk for?
|
Aortic stenosis.
Mitral valve calcification. |
|
|
What is the problem with having a bicuspid aortic valve?
|
Can develop AS or AR with time.
Congenital in 1-2% of the population. Frequent cause of symptomatic aortic stenosis in young patients. Associated with other congenital abnormalities (especially coarctation) in 20% of cases. 80% of coarctation patients have BAV. |
|
|
What does aortic stenosis sound like?
|
Mid to late peaking systolic ejection murmur.
Crescendo-decrescendo. The later in systole the peak is heard the worse the lesion is.When the stenosis is severe enough, you can no longer hear the second heart sound. |
|
|
What is the pressure relationship between the LV and aorta in the systole of an aortic stenosis patient?
|
There is a bigger difference than in a normal heart.
|
|
|
What is the etiology of aortic stenosis?
|
In a young patient think
congenital: Bicuspid (2% of population) 3:1 male:female distribution Co-existing coarctation (6% of patients) Rarer causes include unicuspid valve, Sub-aortic stenosis (Discrete or Diffuse aka Tunnel) In a middle aged patient (4th and 5th decade) think bicuspid or rheumatic disease. In an old patient (6th, 7th, 8th decade) think degenerative —MOST COMMON aortic stenosis. |
|
|
Rheumatic aortic stenosis is usually associated with...
|
Concurrent mitral valve disease.
|
|
|
How fast does aortic stenosis progress?
|
1 cm^2/year
|
|
|
What triad of symptoms is classically associated with aortic stenosis?
|
angina, syncope, heart failure
(Also GI bleeding from AVMs) Symptom development is correlated with survival. Angina (if untreated): 5 year survival Syncope: 3 year survival Heart failure: 2 year survival |
|
|
What physical signs are associated with aortic stenosis?
|
“Diamond” shaped, harsh, systolic crescendo-decrescendo ejection murmur.
Usually heard best at RUSB and radiates to carotids. Can also radiate to the apex and mimic mitral regurgitation (the Gallavardin phenomenon) Paradoxical S2 (delayed left ventricular emptying) S4 (with left ventricular hypertrophy) S3 (with left ventricular failure) Intensity of murmur DOES NOT predict severity. Presence of thrill DOES NOT predict severity. Decreased, delay & prolongation of pulse amplitude. Carotid pulsations characterized by delayed and weakened upstroke (the pulsus parvus et tardus) |
|
|
How do we discern the carotid sounds of aortic stenosis from carotid stenosis?
|
Listen all the way up to the mandible.
Aortic stenosis should gradually disipate. |
|
|
How do we classify the severity of aortic stenosis?
|
Normal aortic valve area:
2-3 cm^2 Mild AS >1.5 cm^2 Moderate AS 1.0-1.5 cm^2 Severe AS <1.0 cm^2; (mean gradients >50 mmHg) |
|
|
How do we treat aortic stenosis?
|
Asymptomatic patients—follow expectantly
Endocarditis prophalaxis Annual echo with severe AS or when symptoms change. Patient education regarding symptoms Surgery in patients with severe, symptomatic aortic stenosis or who need open heart for other reasons. |
|
|
What are the causes of aortic insufficiency?
|
Bicuspid aortic valves, especially when associated with aortic root dilitation.
Infective endocarditis. Rheumatic heart disease.(usually stenosis but can cause incompetence) Radiation. Subvalvular (subpulmonary aka suprachristal VSD) Myxomatous disease. Marfan’s or other connective tissue disorders. Chronic aortitis (syphilitic). Acute causes: Aortic dissection Infective endocarditis Traumatic |
|
|
What is the effect of aortic regurgitation on the LV?
|
Dilation.
Eccentric Hypertrophy. When the LV starts to dilate, the valve needs to be fixed even if the patient is asymptomatic. |
|
|
How does chronic aortic regurgitation present?
|
May remain asymptomatic or nearly so for a long time. Symptoms occur after considerable cardiomegaly and myocardial dysfunction.
exertional dyspnea, orthopnea, PND. Uncomfortable awareness of the heart beat especially on lying down. Syncope is rare and angina is less common than in patients with AS Diffuse apical impulse. Laterally and inferiorly displaced hyperdynamic heart. Variable thrill over the base, suprasternal notch or carotids. Carotid shudder. S1 is soft, A2 is soft or absent, S3 and S4. Early diastolic decrescendo murmur (high frequency) begins immediately after A2 best heard by the diaphragm, radiates to the apex. Severity of AR correlates with duration of the murmur In severe AR Mid to late diastolic apical decresecendo rumble heard best at right sternal border(Austin Flint murmur). This is a result of leakage from the aortic valve shutting the anterior leaflet of the mitral valve. . |
|
|
What are some physical exam signs of aortic regurgitation?
|
Bobbing of the head (de Mossert’s sign), with every heart beat, waterhammer or collapsing type pulses.
Traube’s sign refers to booming systolic and diastolic sounds over the femoral artery. Durozierz’s sign consists of systolic and diastolic murmur over the femoral artery. Quinke’s sign: capillary pulsations seen in fingertips. Hill’s signs: popliteal systolic pressure exceeding brachial artery systolic pressure by 50-60 mmHg, etc. |
|
|
What are the X-ray findings of aortic regurgitation?
|
LV enlargement which can be massive (cor bovinum).
Dilation of ascending aorta, egg-shell calcification of ascending aorta seen in luetic AR. Valve calcification is uncommon. |
|
|
What is the ideal study for following a patient with aortic regurgitation serially?
|
Echo
|
|
|
Which aortic regurgitation patients do we NOT want to operate on?
|
Asymptomatic patients with preserved LVEF and normal LV size.
We think about operating as soon as ventricle starts getting dilated. |
|
|
Fixed splitting is pathgnemonic for...
|
ASD
|
|
|
What is the etiology of mitral stenosis?
|
Primarily rheumatic fever
(Scarring and fusion of valve) Pure or predominant MS in~40% of rheumatic patients. Rarely congenital. 2/3 female. |
|
|
What causes the symptoms of mitral stenosis?
|
Increase in LA pressure causes pulmonary congestion and edema.
Diastolic murmur because of turbulence across the stenotic valve. |
|
|
What are the valve areas with various mitral stenoses?
|
Normal valve area: 4-6 cm2
Mild mitral stenosis: MVA 1.5-2.5 cm2 Minimal symptoms Mod mitral stenosis MVA 1.0-1.5 cm2 Usually no symptoms at rest. Severe mitral stenosis MVA < 1.0 cm2 |
|
|
What is a common response of the LA to mitral stenosis?
|
Valvular atrial fibrillation.
!!Very high risk for stroke!! |
|
|
What happens to the shape of the LV in mitral stenosis?
|
It becomes rounded.
|
|
|
What are the anatomical and functional features of mitral stenosis?
(picture) |
|
|
|
Syncope is associated with...
|
Stenotic valve lesions.
|
|
|
What are the chronic causes of mitral regurgitation?
|
Myxomatous disease (prolapse)
Rheumatic heart disease Annulo-aortic ectasia Other connective tissue disorders Dilated cardiomyopathy (annular dilation) Ischemia (papillary muscle dysfunction) |
|
|
What are the acute causes of mitral regurgitation?
|
Infective endocarditis
Myxomatous disease (choral rupture, flail mitral valve) Ischemic heart disease (post-infarction papillary muscle rupture) |
|
|
The murmur associated with mitral regurgitation is a..
|
Holosystolic murmur heard best at the apex which radiates to the axilla.
|
|
|
The increased load in chronic mitral regurgitation causes what type of compensation?
|
Left Ventricle Dilates AND Hypertrophies
Increased Stroke volume (in a sense) Left Atrium also dilates |
|
|
What is the natural course of chronic mitral regurgitation?
|
Tolerated well
Long A-Symtomatic (or minimal Sx)–10 years or More Mild MR may have Normal life span. BUT If Irreversible LV Dysfunction then MVR is no help. |
|
|
What are the physical findings of mitral regurgitation?
|
Apical impulse, laterally displaced and hyperdynamic.
!Pan – systolic murmur in apex, axilla right precordium or back! S-1: Normal or decreased S-2: May be wide split (A2 early) S-3: Palpable S-4: Rare in severe chronic MR (dilated LA, AF) Normal until very late: JVD |
|
|
What is mitral valve proplapse?
|
Systolic displacement of the mitral leaflet into the left atrium of at least 3 mm beyond the mitral annulus in the parasternal or apical long-axis view.
Mitral regurgitation. Thickened or myxomatous mitral leaflets. Typically have symptomatic PAC's and unexplained chest pain. Midsystolic click. |
|
|
What are the characteristics of Normal, Physiologic Valvular Regurgitation?
|
Always trivial or at most mild
Usually central Valve anatomically normal appearing. |
|
|
What is the risk of being anticoagulated for a prosthetic valve?
|
About 1%/ patient year hemorrhage.
|
|
|
NOT on test!
What are the current prosthetic mechanical valves? |

|
|
|
NOT on test.
What are the current tissue prosthetic valves? |

|
|
|
What normal changes occur in the CV system as we age?
|
Increased stiffness of the left ventricle and arteries.
Decreased maximal heart rate and cardiac output during exercise. Effectively this means substantial reduction in CV reserve. |
|
|
What CV diseases are nearly unique to the elderly population?
|
Diastolic heart failure and calcific aortic stenosis.
|
|
|
What happens to blood pressure as we age?
|
Systolic pressure goes up but diastolic pressure remains pretty constant.
|
|
|
What is the first-line antihypertensive in the elderly?
|
Thiazide diuretic
(with lifestyle changes) Start low, titrate slowly. Monitor closely for adverse effects, especially orthostatic hypotension. Caution regarding pseudohypertension (mismeasuring with cuff and scope) from stiff arteries. Goal BP: same as in younger patients |
|
|
How do we calculate attributable risk?
|
Prevalence*risk ratio
|
|
|
What are the "typical" atypical symptoms in elderly patients with MI?
|
Confusion
Dyspnea Lassitude New onset CHF New arrhythmia Syncope Stroke Sudden death |
|
|
At what age does the CVD risk for men equal that of women?
|
Cardiovascular risk for women rises after menopause and becomes similar to men by age by 70.
|
|
|
What kind of relationship exists between the risk of CVD and BP?
|
Continuous, consistent, and independent of other risk factors.
Each increment of 20/10 mmHg doubles the risk of CVD across the entire BP range starting from 115/75 mmHg. Also sometimes synergistic with other risk factors. |
|
|
How do we stage hypertension?
|
Normal:
<120 AND <80 Prehypertension: 120–139 or 80-89 Stage 1 hypertension: 140–159 or 90-99 Stage 2 hypertension: >=160 or >=100 |
|
|
Using too small a cuff produces a BP reading that is...
|
Artificially high
and vice versa. |
|
|
For people over age 50, which blood pressure measurment is a bigger risk factor?
|
Systolic
|
|
|
Persons who are normotensive at age 55 have a life time risk of developing hypertension of...
|
90%
|
|
|
When do we use two or more antihypertensives?
|
If BP is >20/10 mmHg above goal.
|
|
|
What does BP typically do at night?
|
BP drops by 10 to 20% during the night; if not, signals possible increased risk for cardiovascular events.
|
|
|
What is the recommended goal of antihypertensive therapy?
|
BP <140/90 mmHg
or BP <130/80 mmHg in patients with diabetes or chronic kidney disease. |
|
|
People with a tendency to alcohol excess will often have an elevated...
|
Diastolic BP
|
|
|
What are the guidelines for treating HTN?
(picture) |
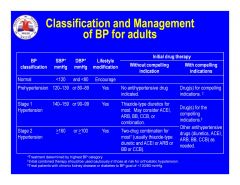
|
|
|
Why are serum potassium and creatinine monitored 1–2 times per year in patients on antihypertensives?
|
To monitor potassium wasting in diuretics and watch for hyperkalemia on an ACE inhibitor.
|
|
|
What are good antihypertensive drug options for patients with heart failure?
(compelling indication) |
THIAZ, BB, ACEI, ARB, ALDO ANT (Spironolactone)
|
|
|
What are good antihypertensive drug options for patients post MI?
(compelling indication) |
BB, ACEI, ALDO ANT (Spironolactone)
|
|
|
What are good antihypertensive drug options for patients with high risk of CAD?
(compelling indication) |
THIAZ, BB, ACE, CCB
|
|
|
What are good antihypertensive drug options for patients with diabetes?
(compelling indication) |
THIAZ, BB, ACE, ARB, CCB
|
|
|
What are good antihypertensive drug options for patients with chronic kidney disease?
(compelling indication) |
ACEI, ARB
|
|
|
What are good antihypertensive drug options for patients that require recurrent stroke prevention?
(compelling indication) |
THIAZ, ACEI
|
|
|
What minority population has the highest prevalence of HTN?
|
African americans.
They are generally less renin sensitive and do better with diuretics and CCB's. |
|
|
When treating hypertension in patients with PAD, we typically also give...
|
Aspirin
|
|
|
When someone has postural hypotension, we need to be especially careful with the antihypertensives...
|
ACE inhibitors.
Afterload reduction can exacerbate the problem. |
|
|
Which antihypertensives are contraindicated in women who are pregnant or have intentions of becoming pregnant?
|
ACEI and ARBs contraindicated in pregnancy.
Pregnant women with HTN should be followed carefully. Methyldopa, BBs, and vasodilators, preferred for the safety of the fetus. Oral contraceptives may increase BP in contrast to HRT which does not. |
|
|
Which antihypertensive is associated with angioedema?
|
ACE inhibitors
|
|
|
Where do the various diuretics act in the nephron?
|
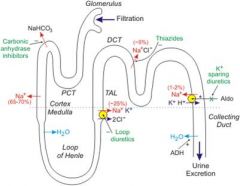
Loop diuretics:
Ascending limb of the loop of Henle (Still in the medulla). Block the exchange of Na+, K+ and Cl- . Very powerful diuretics. Thiazides: Block ion exchange in the distal convoluted tubule. (In the cortex) Less potent than loop diuretics. K+ sparing diuretics (aldosterone anatagonists): End of the nephron. Right before it re-enters the medulla and becomes the collecting duct. Less potent than thiazides. |
|
|
What are the most commonly prescribed thiazide diuretics in the US?
|
HCTZ
(12.5-25mg) Chlorthalidone (12.5-50mg) >25mg ill advised Metolazone (most potent, 2.5-10mg) All PO drugs, chlorthiazide is IV equivalent but rarely used. HCTZ has slightly shorter T1/2 than others (12-18hours vs 24) Can cause hypovolemia, hypokalemia, hypomagnesemia, hyponatremia, HYPERglycemia, gout and rash (contain sulfur). May cause vasodilation after several weeks (double whammy) |
|
|
What loop diuretics are available in the U.S?
|
Furosemide (shortest acting)
Bumetanide Torsemide Ethacrynic acid (does not contain sulfur) Can cause hypovolemia, hypokalemia, hypomagnesemia, hyponatremia, HYPERglycemia, gout and rash (most contain sulfur). Not good antihypertensives because of short half life. Also do not vasodilate like thiazides. Better than thiazides for creatinine clearance <30. |
|
|
What K+ sparing diuretics are available in the U.S?
|
Aldosterone antagonists:
Spironolactone 12.5-50mg (Hyperkalemia, Gynecomastia) Eplerenone 25-50mg (Hyperkalemia) Non-aldosterone-antagonists: Triamterene Amiloride (both can cause hyperkalemia and rash) Very weak diuretics. Sometimes given with amphotericin B antifungal to preserve potassium. |
|
|
The suffix -sartan means..
|
Angiotensin receptor blocker (ARB)
|
|
|
The suffix -pril means...
|
ACE inhibitor.
|
|
|
What does the relatively new antihypertensive drug Aliskiren do?
|
Directly inhibits Renin.
|
|
|
The peak/trough ratio of a once a day ACE inhibitor tells us...
|
What percentage of blood pressure reduction is found after 24 hours.
|
|
|
What is the shortest acting ACE inhibitor?
|
Captopril
TID or QID. Great in critical care setting but bad for outpatient. |
|
|
Enalapril is used once a day for hypertension control but twice a day for...
|
Heart failure or prevention of vascular complications.
Only ACE inhibitor available IV. |
|
|
What are the contraindications for ACE inhibitors and company (ARB's and aliskerin)?
|
Bilateral Renal Artery Stenosis
Pregnancy Angioedema to other ACEIs Hyperkalemia (K+>5meq/L) Can cause an increase in serum creatinine and nephrotoxicity. Paradoxically thouhg, they can be used to halt progression of kidney disease. ACE inhibitors can cause a dry non-productive cough. ARB's do not do this. |
|
|
What are the major side effects of beta blockers?
|
Drowsiness, lethargy, confusion, bronchoreactive events, AV nodal blockade.
|
|
|
What is the often forgotten effect of beta blockers?
|
They block renin receptors by binding beta receptors in the kidneys.
|
|
|
Which beta blocker has a high lipid solubility and why is this important?
|
Propranolol.
Makes it across the blood brain barrier quite welland has more CNS effects. |
|
|
The ideal beta blocker for cardiac use only is...
|
Highly beta 1 selective.
Low lipid solubility. |
|
|
Labetalol and carvedilol are useful drugs because...
|
They also have alpha effects which vasodilate and reduce blood pressure.
|
|
|
Most commonly prescribed beta blockers are...
|
Metoprolol.
(25-100mg bid, B1 selective, moderate lipid solubility) Labetalol (100-300mg bid, non-selective, moderate lipid solubility) Carvedilol (3.125-25mg bid, non-selective, moderate lipid solubility) |
|
|
What are common adverse reactions of beta blockers?
|
Drowsiness, Lethargy
Bradycardia/ Heart Block Bronchoreactive Events May mask signs of hypoglycemia |
|
|
What are the contraindications for beta blocker use?
|
Symptomatic Bradycardia/Hypotension
Decompensated HF Use with caution: asthma, diabetes (only sign of hypoglycemia not masked by beta blockers is diaphoresis) |
|
|
What is the effect of diltiazem and verapamil on the heart?
|
Decrease AV nodal conduction.
Decrease HR. Decrease contractility. Minimal effect on vascular resistance. Verapmail reduces HR and contractility more. Not typically use for HTN unless there are Arrhythmias too. |
|
|
What is the effect of dihydropyridine CCB's on the vasculature?
|
Vasodilation
All end in -pine. |
|
|
What are terazosin, doxazosin and prazosin?
|
Pure alpha 1 blockers.
Reserved for unique cases: males with benign prostatic hypertrophy. 1st dose syncope, dizziness, lethargy. ↑ in CV events when used as monotherapy. |
|
|
What are clonidine and methyldopa?
|
Alpha2-receptor Agonists
(reduce sympathetic outflow) Add-on therapy with diuretics. Available as transdermal preparation. Nasty (not dangerous) side effects. |
|
|
What are Hydralazine and Minoxidil?
|
Vasodilators.
Use with diuretic and beta-blocker to counteract compensatory changes. Minoxidil causes sodium retention and hair growth. |
|
|
Maximum heart rate is about...
|
220-age
|
|
|
Where is the accessory pathway in WPW?
|
Bundle of kent.
|
|
|
Bicuspid aortic valve have an association with....
|
Coarctation.
|
|
|
How much fluid should there normally be between the viseceral and parietal (fibrous) pericardium?
|
10-50cc
|
|
|
Congenital absence of pericardium is usually an incidental diagnosis on CT, MR or CXR. It is associated with...
|
Atypical chest pain or sudden death.
This is unlikely a causal relationship though. |
|
|
Percardial cysts are quite common and usually don't require unless...
|
They impinge on the heart or an adjacent structure.
|
|
|
The most common etiology for infectious pericarditis is...
|
Viral
(coxsackie A & B, echovirus, mumps, adenovirus, HIV) Bacterial causes are very bad and include: !Mycobacterium tuberculosis!, Pneumococcus, Streptococcus, Staphylococcus, Legionella Fungal: histoplasmosis, coccidioidomycocis, candidiasis, blastomycosis |
|
|
The mechanism of uremic pericarditis in dialysis patients...
|
Is not currently understood.
|
|
|
What diseases are associated with hypersensitivity pericarditis?
|
Systemic lupus erythematosus
Rheumatoid arthritis Scleroderma Acute rheumatic fever |
|
|
What drugs can cause hypersensitivity pericarditis?
|
procainamide
hydralazine isoniazid others |
|
|
What is Dressler's syndrome?
|
Late presenting (days to weeks) hypersensitivity pericarditis after an MI.
|
|
|
How does acute pericarditis typically present?
|
SUDDEN severe retrosternal or left precordial pain:
May refer to back or trapezius. May be preceded by low-grade fever. May be pleuritic or positional (worse supine). With viral pericarditis, usually 1-2 weeks after “viral illness”. Resting tachycardia Low-grade fever (if infectious etiology) Pericardial friction rub. (Classically triphasic but can be any) ECG: Diffuse ST elevation early Depression of PR segment T inversion late Low voltage in QRS (if large) Electrical alternans (if large) A-Fib Elevated Sed rate and WBC. Echo can sometimes show pericardial effusion but not always. |
|
|
Some of the features that help us discern early pericarditis from STMI on ECG are...
|
Pericarditis elevations don't typically conform to an anatomical distribution like MI and generally appear in the epicardial leads (I, II, aVL, aVF, V3-V6). They are thus said to be are more diffuse.
Endocardial leads aVR, V1 and V2 will also show ST depression in percarditis Camel hump type shape in the ST segments is also suggestive of pericarditis. PR depression is commonly seen in pericarditis. |
|
|
What ST changes are charcteristic of stage II of pericarditis?
|
Normalization
In stage 3 we start seeing ST depressions and T inversions. |
|
|
How do we treat acute pericarditis?
|
If a significant pericardial effusion is not present, treatment is targeted to symptoms - usually NSAID's.
If peri-infarct pericarditis, may prefer high dose aspirin. Steroids and Colchicine for resistant pericarditis. |
|
|
What type of fluid is found in a pericardial effusion?
|
Serosanguinous effusions - neoplasm, Tb, uremia, idiopathic, non-infectious cause.
Hemopericardium - trauma, myocardial rupture, aortic dissection, coronary artery rupture. Purulent effusion - secondary to direct invasion of the pericardial space by infectious organisms. |
|
|
What imaging findings do we expect with a pericardial effusion?
|
Cardiomegaly or “water-bottle” shape on CXR.
ECG - low voltage Echocardiography - imaging procedure of choice. |
|
|
How does cardiac tamponade present?
|
Presentation may mimic heart failure: Dyspnea on exertion and orthopnea
Tachycardia and tachypnea Jugular venous distention Hypotension and poor peripheral perfusion Shock and profound hemodynamic collapse Pulsus Paradoxus (>10mmHg) Can range from very mild to cardiogenic shock. Beck's triad: Hypotension, JVD, Muffled heart sounds. Tx: Fluids, pressors, percardiocentesis and/or pericardectomy. |
|
|
What is constrictive pericarditis?
|
Results from dense fibrosis, adhesion of the parietal/ visceral layers and eventually calcification from any cause of pericarditis including radiation, prior infectious pericarditis and cardiac surgery.
Much more chronic than tamponade. RHF symptoms. Prominent Y descent with JVP. “Kussmaul’s sign” - exagerated jugular venous filling with inspiration. “pericardial knock”- characteristic abnormal sound in diastole. |
|
|
What do we look for on CXR to in restrictive pericarditis?
|
White calcification line around the heart. Can see on CT and MRI too along with pericardial thickening.
Echocardiography: signs of ventricular interdependence; respiratory variation in left and right ventricular filling. Prognosis is poor but we try stripping the pericardium surgically. |
|
|
The main hemodynamic difference between tamponade and constrictive pericarditis is that...
|
In tamponade, the heart is affected throughout diastole.
In restrictive pericarditis it is only affected towards the end of diastole. |
|
|
The most common manifestation of blunt trauma to the heart is...
|
Cardiac contusion.
New arrhythmias or ECG changes. LV wall motion abnormality on echo. |
|
|
What is an aortic transsection?
|
Shear forces in decceleration injuries can cause damage to the descending aorta.
Clinical manifestations can be delayed (~1month) and resemble an aneurysm. |
|
|
What is the effect of hyperthyroidism on the heart?
|
Hyperkinetic cardiovascular state with fall in systemic vascular resistance, increase in cardiac output, and enhanced left ventricular emptying; may develop atrial fibrillation.
|
|
|
What is the effect of hypothyroidism on the heart?
|
Hypertension, bradycardia, and pericardial effusions.
|
|
|
What is the effect of pheochromocytoma on the heart?
|
Catecholamine induced myocardial necrosis.
|
|
|
What part of the heart do metastatic carcinoid processes usually affect?
|
Right heart.
|
|
|
What are the three major classes of cardiomyopathies?
|
Dilated Cardiomyopathy:
Systolic dysfunction Reduced ejection fraction Restrictive Cardiomyopathy: Diastolic dysfunction Ejection fraction may be normal Hypertrophic Cardiomyopathy: A genetic condition, not really a class but a single disease. |
|
|
What is the most common non-ischemic cardiomyopathy etiology?
|
Idiopathic dilated cardiomyopathy.
May be the result of viral myocarditis or toxic myocarditis but etiology no longer apparent at time of presentation. 3 times more common in african american males. Genetic predisposition, sometimes familial. Heart biopsy is not diagnostic. No specific treatment - general CHF treatment. |
|
|
What causes viral myocarditis?
|
Multiple viruses but most commonly enterovirusses (Coxsackie B - 50% of all cases).
Immunologic mechanism - develops weeks after the original infection. More aggressive and fulminant in infants and pregnant women. |
|
|
Lyme disease spirochete myocarditis is associated with...
|
Heart block.
|
|
|
What is an important protozoal cause of cardiomyopathy in South America?
|
Trypanosomiasis
(Chaga’s Disease-T. cruzi) |
|
|
How does peripartum cardiomyopathy differ from idiopathic cardiomyopathy.
|
Clear relationship to recent pregnancy with a high risk of relapse on subsequent pregnancies.
Embolic phenomena are more common. Better Prognosis (1/3 recover completely, 1/3 stabilize, 1/3 progress) Evidence of inflammation on heart biopsy is actually a good prognostic sign. |
|
|
What are the clinical risk factors for peripartum cardiomyopathy?
|
Multiparity
Advanced maternal age Twin pregnancies Pre - eclampsia |
|
|
How do we treat peripartum cardiomyopathy?
|
Onset is typically from third trimester up to several months post partum.
No Specific therapy (conventional heart failure therapy ) except anticoagulation is important. Surgical sterilization is highly recommended because of the risk of relapse on subsequent pregnancies. |
|
|
What is hypertrophic cardiomyopathy?
|
Cardiac muscle disease characterized by abnormal hypertrophy, mainly involving the ventricular septum.
Histology : Myofibrillar disarray Functional abnormalities: Outflow tract obstruction Diastolic dysfunction Atrial and Ventricular arrhythmias. 50% autosomal dominant. |
|
|
What clinical findings are consistent with hypertrophic cardiomyopathy?
|
Loud fourth heart sound.
Brisk bifid carotid pulse. Harsh systolic ejection murmur like aortic stenosis but doesn’t usually radiate to the neck and it is labile. (varies with maneuvers) Increases on standing, diminishes with raising of the legs. Sometimes also pansystolic murmur (mitral regurg) Echo findings: Assymetric septal hypertrophy. Systolic anterior motion of the mitral valve. Gradient across ventricular outflow tract and likely mitral regurgitation. |
|
|
Whta diseases are considered predisposing to infective endocarditis?
|
Rheumatic heart disease.
Congenital heart disease (PDA, Fallot’s tetralogy, coarct, VSD, ASH) Degenerative heart disease (calcific AS, mitral annular calcification) Mitral valve prolapse (very high risk) |
|
|
What can we say about the epidemiology of native valve infective endocarditis?
|
Native valve endocarditis in now a disease of middle-aged adults, most of whom will have an underlying valvular lesion (e.g., degenerative heart disease) that predisposes to infection.
Rheumatic heart disease and congenital heart disease are increasingly uncommon predisposing lesions in developed countries. IV drug use is a major risk factor for acquisition of infection. |
|
|
How does native valve endocarditis actually occur?
|
The development of native valve IE usually requires 2 concurrent or sequential events→The formation of a nonbacterial thrombus on an endothelial surface AND a transient bacteremia with an organism capable of adhering to a platelet-fibrin matrix.
|
|
|
What are the pathophysiological consequences of endocarditis?
|
Local tissue injury/Valve damage
Local extension of infection Valve ring abscess Myocardial abscesses Pericarditis Distant emboli (bland & septic) Immune complex phenomena |
|
|
Recent data suggest that strep has been replaced as the most common etiological agent of native valve endocarditis by..
|
S.Aureus
80-90+% of cases of native valve IE are caused by gram positive cocci (Staph, Strep, Enterococci) GNRs frequently cause bacteremia but rarely cause IE Strep, enterococci, & CNS cause subacute disease; S. aureus causes acute disease IVDU-associated IE usually due to S. aureus & most often involves tricuspid valve IE is “culture negative” in 5-10% of cases. |
|
|
If I am postulating a diagnosis of native valve infective endocarditis and blood cultures are growing an organism other than a Gram positive coccus, what should I do? .
|
Alternative diagnoses should be strongly entertained
|
|
|
The most important clinical manifestations of infective endocarditis we are looking out for...
|
Fever
Heart murmurs Embolic phenomena Skin manifestations |
|
|
What are Osler's nodes?
|
Tender erythematous nodules in the pulps of the fingertips or toes that are related to endocarditis.
|
|
|
What are some of the major complications of infective endocarditis?
|
Congestive heart failure
Systemic emboli Mycotic aneurysms Renal disease |
|
|
What is the leading cause of death in infective endocarditis?
|
CHF
|
|
|
How do we diagnose infective endocarditis?
|
Diagnosis predicated on compatible clinical syndrome with demonstration of !continuous! bacteremia.
Documentation of presence of valvular vegetations (echo, surgery) desirable but not essential. |
|
|
As defined by the Duke criteria, a definitive diagnosis of infective endocarditis is most convincingly established when ...
|
Appropriate microbiology→2 or more blood cultures obtained at different times that are positive for a Gram positive coccus.
Evidence of endocardial involvement→Demonstration of a valvular vegetation or a new regurgitant murmur via echo. |
|
|
How do we manage infective endocarditis?
|
Prolonged IV course (4-6 weeks) of bactericidal agent.
Careful monitoring of therapy warranted. !document clearance of bacteremia! Surgery: Indicated in face of defined complications. Prior duration of ATB Rx not a determining variable. Less than 5% of prosthetic valves inserted during active IE become infected. |
|
|
What are the complications that warrant surgical intervention in infective endocarditis?
|
Uncontrolled heart failure.
Multiple embolic episodes. Persistent bacteremia despite appropriate antibiotics. Fungal (i.e., Candida) endocarditis Infection due to a MDR organism (ie, VRE) Development of heart block, bundle branch block, or purulent pericarditis Prosthetic valve dehiscence or obstruction Relapse following “adequate” trial of antibiotic therapy |
|
|
What is early prosthetic valve endocarditis?
|
< 2 mos of surgery
Secondary to intraop or periop contamination. Valvular dysfunction with perivalvular leak common Staph, GNRs, fungi Surgery! |
|
|
What is late prosthetic valve endocarditis?
|
> 2 mos post-surgery
Secondary to seeding of valve during bacteremia from non-cardiac site S. aureus, coag neg staph, Strep sp. Medical cure possible though surgery freq’ly necessary |
|
|
As compared to native valve IE, PVE is associated with a more...
|
Varied microbiology.
Frequent metastatic complications. Greater need for surgical intervention, and a poorer outcome. |
|
|
What is the current stance on prophylaxing patients for procedure with a potential to cause infective endocarditis?
|
The focus on prevention of IE has shifted from antibiotic prophylaxis before “risk procedures” to optimizing dental hygiene and health in recognition of the fact that only an extremely small number of cases of IE might be prevented by antibiotic prophylaxis even if prophylaxis is 100% effective.
Still done though. |
|
|
The majority of prosthetic valve endocarditis cases are...
|
Late PVE (~67-85% of cases)
> 2 mos post-surgery. Seeding of valve during bacteremia from non-cardiac site. |
|
|
What percentage of coronary artery stenosis does there need to be to produce ischemic symptoms?
|
75%
|
|
|
What is found in an infarct at various post infarction stages?
|
<24 hours Early acute
Thin wavy fibers and contraction bands histologically. 1-6 days Acute: PMN's 1-3 weeks Organizing: Granulation tissue. Acute and chronic inflammation; Macrophages and Fibroblasts. >3 months remote: Collagen |
|
|
What is the time interval from myocardial ischemia to irreversible damage?
|
20-40 minutes
|
|
|
When does postMI rupture (free wall, septal or papillary) typically happen?
|
First week after MI.The acute phase.
Usually latter part of the week. This is when there is maximal necrosis. |
|
|
When is post-MI aneurysm most likely to happen?
|
Can happen any time from the acute phase onwards (24 hours and up) but most likely in the organizing stage. (1-3 weeks)
|
|
|
When does post-MI CHF usually happen?
|
Any time after the MI.
|
|
|
Whe are post MI arrythmias most likely to happen?
|
Early acute stage. <24hours
|
|
|
When are post MI mural thrombus and pericarditis most likely?
|
Acute and organizing stages
(24 hours to 3 weeks) Pericarditis is more common in organizing stage than acute stage. |
|
|
What can cause pure right sided failure?
|
intrinsic lung disease.
(cor pulmonale) Usually not heart disease. |
|
|
What disease are Aschoff bodies & Anitschkov myocytes associated with?
|
Acute rheumatic fever
Verrucae is the name of the "vegetations" seen on the valve, |
|
|
What valves does rheumatic heart disease usually affect?
|
Mitral > Aortic > Tricuspid > Pulmonic
Mitral alone 48%, Mitral + aortic 42% R. sided valves only rarely; TR usually due to CHF |
|
|
How does chronic rheumatic heart disease affect mitral valve morphology?
|
Thickening of valve leaflet, especially along lines of closure
Fusion of commissures Thickening, shortening and fusion of chordae tendinae Result is mitral stenosis |
|
|
Fish mouth deformity of the aortic valve is associated with...
|
Rheumatic heart disease.
|
|
|
Acquired aortic stenosis in middle age is almost always..
|
Rheumatic heart disease.
|
|
|
What are the major etiologies of aortic insufficiency?
|
Rigidity - rheumatic, degenerative
Destruction – microbial endocarditis Collapse - prolapse through VSD, or due to myxomatous degeneration Disease of AoV ring: Cystic medial degeneration Marfan Syndrome Dissecting aneurysm (medial degeneration or hypertension) Syphilitic aortitis Ankylosing spondylitis |
|
|
What is the epidemiology of infective endocarditis in IV drug users?
|
Right sided valve endocarditis more common in addicts than in non-addicts
(but still the majority of cases in addicts are left sided) |
|
|
What is marantic endocarditis?
|
Nonbacterial thrombotic endocarditis.
Small (1-5 mm), sterile fibrin & Plt thrombi loosely adherent to valves (lines of closure), mainly MV Due to systemic hypercoagulability May embolize systemically |
|
|
What is Libman-Sacks endocarditis?
|
A non-infective endocarditis (valvulitis) occurring in SLE & anti-phospholipid syndrome patients.
MV & TV most commonly affected Microscopic: fibrinoid necrosis Fibrinous vegetations on either side of valve Valve deformations may occur |
|
|
Carcinoid heart disease causes...
|
Mainly right-sided valvular lesions.
Elaboration by Argentaffinomas of bioactive products including serotonin, bradykinin, histamine, PG, etc. |
|
|
What are the epidemiological features of mitral valve proplapse?
|
Sex: F:M:: 6:4
Age: 20 - 40 |
|
|
What are the clinical features of mitral valve proplapse?
|
May be asymptomatic.
Mid-systolic click Atypical chest pain Dyspnea Fatigue Pysch. manifestations Can secondary infective endocarditis and sudden cardiac death. Morphology of MVP includes stretched, redundant valves, usually mitral but sometimes also tricuspid and semilunar valves. |
|
|
What is the normal thickness of the LV wal?
|
<1.5cm
Healthy heart weight typically 300-350g. |
|
|
Atheroscelrosis does not ever occur in the pulmonary artery unless...
|
There is pulmonary hypertension.
|
|
|
What are the clinical features of hypertrophic cardiomyopathy? (HOCM)
|
Impaired ventricular compliance
Pump failure Systolic murmur Dysrhythmias Sudden death Key histological feature is myofiber disarray. Familial in 50% (AD, variable penetrance) |
|
|
How does amyloidosis affect the heart?
|
Infiltrates myocardium and cause a restrictive cardiomyopathy.
Characteristic Congo Red staining. Can mimic ischemic heart disease in symptoms (failure, EKG changes) Can have amyloid limited to heart and vessels . |
|
|
Major risk factors for CVD include hypertension, dyslipidemia, diabetes, smoking, and family history. Ideal prevention and/or control of the first four factors can reduce CVD rates by approximately what proportion in the United States?
|
75%
|
|
|
What population has the highest YPLL (years of potential life lost) form CV disease?
|
African americans
|
|
|
What is the single most important predictive risk factor of CHF?
|
Age
!!Hypertension is the most important "biological predictor"!! |
|
|
Prevalence is equal to...
|
Incidence*duration.
|
|
|
How does ethnicity affect CVD risk?
|
Black race associated with increased mortality from IHD
Hispanic and Asians less IHD mortality than Whites. |
|
|
What is the “Stroke Belt”?
|
Southern states have more Stroke than others regions.
|
|
|
AHA Guidelines for Primary and Secondary Prevention include...
|
Cessation of smoking
Lipid assessment & treatment Blood pressure control Diabetes management Aspirin for appropriate candidates |
|
|
What factors affect myocardial oxygen demand?
|
Heart rate
Contractility LV filling pressure Afterload |
|
|
Angina precipitated with exertion is...
|
Stable angina
|
|
|
How does percent diameter reduction in the coronaries correlate with ischemic symptoms?
|
<50% should not cause significant mechanical obstruction of coronary blood flow
50-75% may cause a relative obstruction if myocardial oxygen demand is severely increased >75% is considered a severe obstruction that will mechanically impede coronary flow even at low levels of activity >90% is a critical stenosis ! |
|
|
What is unstable angina?
|
New onset angina less than two months
Angina at low levels of activity Angina at rest Increase in frequency or severity of previously stable angina All anginal type rest pain without evidence of myocardial infarction |
|
|
What drugs reduce myocardial oxygen demand?
|
beta blockers
nitrates ACE inhibitors |
|
|
What drugs reduce coronary artery spasm?
|
CCB's
Nitrates |
|
|
By current clinical definitions, unstable angina turns into acute MI when...
|
It occurs and persists at rest in spite of optimal medical therapy.
Pain duration greater than 20 minutes - may come and go but is persistent. Accompanying EKG changes show transmural infarction. |
|
|
On what time scale does coronary revascularization need to take place?
|
30 minutes - reversible ischemia
1-3 hours - window of opportunity >6 hours - high chance of irreversible necrosis |
|
|
What are the three main goals of treating ischemic heart disease?
|
Relieve angina
Prevent myocardial infarction Promote longevity |
|
|
What vessels can be used for CABG?
|
Saphenous vein graft (SVG)
Internal mammary artery (LIMA or RIMA) Free radial artery Gastroepiploic artery |
|
|
At what rate do saphenous vein grafts close?
|
~5%/year
|
|
|
What are sirolimus and paclotaxil?
|
Antiproliferative agents that are used on stents to prevent intimal hyperplasia.
Sirolimus is in the "Cypher" stent. Paclitaxil is in the "Taxus" stent. |
|
|
How does late restenosis with drug eluting stents compare to bare metal stents?
|
Significantly better <10%
Compared to the 20+% of bare metal stents. |
|
|
What is late stent thrombosis?
|
potentially life threatening complication of PCI/stenting.
!!0.6%/year!! Suspected causes are discontinuation of antiplatelet therapy, late incomplete apposition, polymer hypersensitivity/inflammation and delayed endothelialization. |
|
|
CABG is genrally better for...
|
Severe obstruction multivessel disease.
|
|
|
So how do we decide what to do with ischemic patients?
|
Stenoses of 50-75%:
1,2,3 vessel - try medical therapy If angina persists consider PCI-DES or CABG Stenoses of >75%: 1,2,3 vessel - individualize based on lesion morphology, LV function – PCI-DES vs CABG 3 vessel - diffuse, poor LV, diabetic - CABG left main - CABG |
|
|
Embryologically valve tissues are derived from...
|
Muscle tissue.
|
|
|
What is the overall incidence of congenital heart defects?
|
1:100 live births
|
|
|
How long does it take for the pulmonary bed of a newborn to become fully "relaxed"?
|
4-8 weeks.
Most shunt lesions do not have any physical findings or sx until pulm resistance drops “enough” |
|
|
What is the most common reason for CHF in pediatrics?
|
Pressure &/or volume load .
Intrinsic muscle problems are possible but not likely. |
|
|
What signs and symptoms of CHF are not commonly seen in kids?
|
Peripheral edema
Ascites |
|
|
What is the single most common congenital heart disease?
|
VSD
|
|
|
What determines the presentation and physical exam findings in VSD?
|
The size of the hole
|
|
|
What are the indications for surgically correcting a VSD?
|
Huge hole with uncontrollable CHF
Hole large enough to cause pulm HTN Large heart later, other complications. |
|
|
What are the exam findings of a small VSD?
|
Child thriving
Heart not large Murmur begins at onset of systole (with S1) and continues as long as a pressure gradient exists between left & right ventricles = holosystolic Loudness & pitch vary with size & location. Louder sound means smaller hole. |
|
|
What is the second most common congenital heart defect?
|
ASD
|
|
|
What are the findings of ASD?
|
Palpably enlarged right ventricle
Murmurs from normal valves with too much blood flow (relative stenosis) Pulmonary flow Tricuspid flow Widely (“fixed”) split second sound. Almost never causes symptoms. If hole large, closure recommended by pre-school age to prevent mortality and morbidity in early adulthood. |
|
|
What are the physical findings of PDA?
|
Size of defect determines symptoms and physical findings
Flow occurs as long as Ao pressure > PA pressure (ie continuously) Typically - large heart with continuous “machinery” type murmur best heard under left clavicle; bounding pulses |
|
|
What are the physical findings of congenital pulmonary stenosis?
|
Usually no symptoms unless severe PS in a newborn
RV may feel enlarged if very thick May have palpable thrill if severe Murmur occurs as blood ejected across valve orifice = systolic ejection murmur Heard best over pulm art = LUSB Usually radiates into lung fields |
|
|
What are the cyanotic heart defects?
|
Hey
Harry, This Turkey Tastes Terrible ! Hypoplastic right heart syndrome: Tricuspid atresia; pulm atresia Hypoplastic left heart syndrome. Transposition of the great arteries Total anomalous pulm venous return (TAPVR): (Transposition of the pulmonary veins) Tetralogy of Fallot Truncus arteriosus |
|
|
What is the most common cyanotic heart defect?
|
Tetralogy of Fallot.
Combination of 4 abnormalities due to conal septum malalignment: Large VSD (non-restictive to pressure) Pulmonary stenosis Aorta over-rides the VSD Right ventricular hypertrophy Cyanosis varies by the amount of obstruction of blood flow to lungs: May be minimally cyanotic (pink tet) if PS very mild May be critically cyanotic (blue tet) if PS severe (ductal dependant). |
|
|
What are the exam findings in tetralogy of fallot?
|
Palpably enlarged right ventricle.
May have thrill. Murmur is produced by the PS, not by the VSD. Systolic ejection murmur radiates into lung fields Usually can’t hear pulmonary closure (single S2) due to stenosis. |
|
|
What are the charcteristics of transposition of the great arteries?
|
Male dominance (60-70%)
Usually large well-grown infants. Cyanotic from the “get-go” Must have mixing of the circulations in order to survive ASD or non-restrictive PFO PDA Some have a VSD Keep PDA open with prostaglandin E1 or balloon atrial septostomy sometimes required emergently. |

