![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
44 Cards in this Set
- Front
- Back
|
Embryonic Structure Truncus arteriosus (TA) Bulbus cordis Primitive atria Primitive ventricle Primitive pulmonary vein Left horn of sinus venosus (SV) Right horn of SV Right common cardinal vein and right anterior cardinal vein |
Gives Rise To Ascending aorta and pulmonary trunk Smooth parts (outflow tract) of left and right ventricles Trabeculated part of left and right atria Trabeculated part of left and right ventricles Smooth part of left atrium Coronary sinus Smooth part of right atrium Superior vena cava |
|
|
The heart is the first functional organ in vertebrate embryos |
Beats spontaneously by week 4 of development |
|
|
Heart morphogenesis Cardiac looping |
Primary heart tube loops to establish left-right polarity; begins in week 4 of gestation. Defect in left-right dynein can lead to dextrocardia (right points to the right), as seen in Kartagener syndrome (primary ciliary dyskinesia when accompanied by situs inversus, chronic sinusitis and bronchiectasis) |
|
|
Heart morphogenesis Septation of chambers (Atria) |
Septum primum grows toward endocardial cushions, narrowing foramen primum. Foramen secundum forms in septum primum (foramen primum disappears). Septum secundum forms as foramen secundum maintains right-to-left shunt. Septum secundum expands and covers most of foramen secundum, what is left is the foramen ovale. Remaining portion of septum primum forms valve of foramen ovale. Septum secundum and primum fuse to form atrial septum. Foramen ovale usually closes soon after birth because of high left atrial pressure. Patent foramen ovale caused by failure of septum primum and secundum to fuse after birth. Can lead to paradoxical emboli (venous thromboemboli that enter systemic arterial circulation), similar to those resulting from an atrial septal defect. |
|
|
Heart morphogenesis Septation of chambers (Ventricle) |
Muscular ventricular septum forms. Opening is called interventricular foramen. Aorticopulmonary septum rotates and fuses with muscular ventricular septum to form membranous interventricular septum, closing interventricular foramen. Growth of endocardial cushions separates atria from ventricles and contributes to both atrial septation and membranous portion of the interventricular septum. Ventricular septal defect most commonly occurs in the membranous septum; acyanotic at birth due to left-to-right shunt. |
|
|
Heart morphogenesis Outflow tract formation |
Truncus arteriosus rotates. Neural crest and endocardial cell migrations → Truncal and bulbar ridges that spiral and fuse to form aorticopulmonary septum → ascending aorta and septum.
Conotruncal abnormalities: - Transposition of great vessels - Tetralogy of Fallot - Persistent truncus arteriosus |
|
|
Heart morphogenesis Valve development |
Aortic/pulmonary: derived from endocardial cushions of outflow tract Mitral/tricuspid: derived from fused endocardial cushions of the AV canal
Valvular abnormalities: Stenotic, regurgitant, atretic (tricuspid atresia), or displaced (Ebstein anomaly) |
|
|
Fetal erythropoiesis Occurs in: Yolk sac (3-8 weeks) Liver (6 weeks - birth) Spleen (10-28 weeks) Bone marrow (18 weeks to adult) Young Liver Synthesizes Blood |
Hemoglobin development Alpha Always; Gamma Goes, Becomes Beta Fetal hemoglobin (HbF) = α2γ2 Adult hemoglobin (HbA) = α2β2 HbF has higher affinity for oxygen, this allows it to extract oxygen from maternal HbA across the placenta |
|
|
Fetal circulation Blood in umbilical vein has PO2 = 30 mmHg and is 80% saturated with O2. Umbilical arteries have low O2 saturation. |
3 important shunts: Blood entering through umbilical vein is conducted via the ductus venosus into the IVC to bypass the hepatic circulation. Most oxygenated blood that enters via the IVC is diverted through foramen ovale and pumped out the aorta. Deoxygenated blood entering the RA via the SVC goes: RA → RV → main PA → patent ductus arteriosus → descending aorta. |
|
|
Fetal circulation At birth infant takes breath; ↓ resistance in pulmonary vasculature causes ↑ LA pressure vs RA pressure; foramen ovale closes (now fossa ovalis); ↑ in O2 and ↓ in prostaglandins → closure of ductus arteriosus |
Indomethacin helps close patent ductus arteriosus (PDA) → DA remnant (ligamentum arteriosum). Prostaglandins E1 and E2 keep PDA open. |
|
|
Fetal-postnatal derivatives Umbilical vein Umbilical arteries Ductus arteriosus Ductus venosus Foramen ovale Allantois Notochord |
Ligamentum teres hepatis (Contained in falciform ligament) Medial umbilical ligaments Ligamentum arteriosum Ligamentum venosum Fossa ovalis Urachus-median umbilical ligament Nucleus pulposus of intervertebral disc |
|
|
Coronary artery anatomy Right coronary artery (RCA) Posterior descending/IV artery (PDA) Acute marginal artery Left main coronary artery (LCA) Left circumflex coronary artery (LCX) Left anterior descending artery (LAD) Left marginal artery |
SA and AV nodes supplied by RCA. Infarct may cause nodal dysfunction. LCX supplies lateral and posterior walls of LV LAD supplies anterior 2/3 of IV septum, anterior papillary muscle, and anterior surface of LV PDA supplies posterior 1/3 of IV septum and posterior walls of ventricles Acute marginal artery supplies RV Coronary occlusion occurs commonly on LAD Coronary blood flow peaks in early diastole. Most posterior part of heart is LV; enlargement can cause dysphagia (due to compression of esophagus) or hoarseness (compression of left recurrent laryngeal nerve) |
|
|
Cardiac output CO = stroke volume X heart rate Mean arterial pressure = CO X TPR MAP = 2/3 diastolic pressure X 1/3 systolic p Pulse pressure = SP - DP PP proportional to SV SV = End diastolic volume - end systolic V |
In early stages of exercise CO is maintained by ↑ HR and ↑ SV. Late stages only by ↑ HR. ↑ PP in hyperthyroidism, aortic regurgitation, arteriosclerosis, obstructive sleep apnea, exercise. ↓ PP in aortic stenosis, cardiogenic shock, cardiac tamponade, advanced heart failure. |
|
|
Cardiac output variables Stroke volume Contractility |
Stroke Volume affected by Contractility, Afterload and Preload (SV CAP). ↑ SV when ↑ C, ↑ P and ↓ A.
Contractility ↑ with: catecholamines (↑ activity of Ca pump), ↑ intracellular Ca, ↓ extracellular Na (↓ activity of Na/Ca exchanger), digitalis (blocks Na/K pump → ↑ intracellular Na → ↓ Na/Ca exchanger → ↑ intracellular Ca) Contractility ↓ with: β1-blockade (↓ cAMP), Heart failure with systolic dysfunction, acidosis, hypoxia/hypercapnia, non-dihydropyridine Ca channel blockers. |
|
|
Cardiac output variables Preload Afterload Ejection fraction
EF = SV/EDV = (EDV-ESV)/EDV |
Preload approximated by EDV; depends on venous tone and circulating blood volume. VEnodilators (e.g., nitroglycerin) ↓ prEload.
Afterload approximated by MAP. Laplace's law: relation of LV size and afterload. LV hypertrophy with ↑ afterload to ↓ wall tension. VAsodilators (e.g., hydrAlazine) ↓ Afterload (Arterial).
ACE inhibitors and ARBs ↓ preload and afterload. Chronic hypertension (↑ MAP) → LV hypertrophy.
Ejection fraction LV EF is an index of ventricular contractility; normal EF ≥ F55%. EF ↓ in systolic HF; EF normal in diastolic HF. |
|
|
Resistance, Pressure, Flow |
Pressure gradient drives flow from high to low pressure. Resistance is directly proportional to viscosity and vessel length and inversely proportional to radius to the 4th power. Arterioles account for most of TPR → regulate capillary flow. Viscosity depends mostly on hematocrit. Viscosity ↑ in: Polycythemia, hyperproteinemic states, hereditary spherocytosis. Viscosity ↓ in anemia. |
|
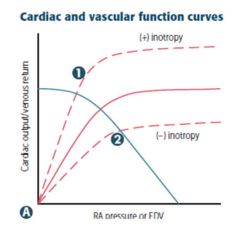
|
Inotropy Changes in contractility → altered CO for a given RA pressure (preload) 1) Catecholamines, digoxin (+) 2) Uncompensated heart failure, narcotic overdose (-) |
|
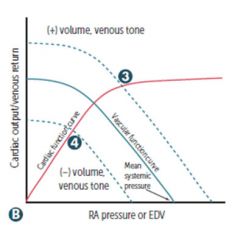
|
Venous return Changes in circulating volume or venous tone → altered RA pressure for a given CO. Mean systemic pressure changes with volume/venous tone. 3) Fluid infusion, sympathetic activity (+) 4) Acute haemorrhage, spinal anesthesia (-) |
|
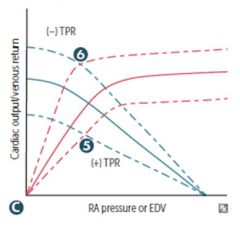
|
Total peripheral resistance Changes in TPR → altered CO at a given RA pressure; mean systemic pressure is unchanged. 5) Vasopressors (+) 6) Exercise, AV shunt (-) |
|
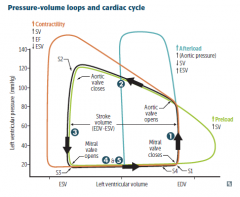
|
Black loop normal cardiac physiology. Phases LV: 1) Isovolumetric contraction: Between mitral valve closing and aortic valve opening; period of highest O2 consumption. 2) Systolic ejection: Between aortic valve opening and closing. 3) Isovolumetric relaxation: Between aortic valve closing and mitral valve opening. 4) Rapid filling: Period just after mitral valve opening. 5) Reduced filling: Period just before mitral valve closing. Orange loop ↑ contractility Green loop ↑ preload Blue loop ↑ afterload |
|
|
Sounds |
S1: Mitral and tricuspid valve closure. Loudest at mitral area. S2: Aortic and pulmonary valve closure. Loudest at left sternal border. S3: In early diastole during rapid ventricular filling. Associated with ↑ filling pressures and more common in dilated ventricles. S4 ("atrial kick"): In late diastole. High atrial pressure. Associated with ventricular hypertrophy. LA must push against stiff LV wall. |
|
|
Splitting |
Normal splitting: Inspiration → ↓ intrathoracic pressure → ↑ venous return to the RV → ↑ RV stroke volume → delayed closure of pulmonary valve. Wide splitting: Seen in conditions that delay RV emptying (pulmonic stenosis, RBBB). Delay in RV emptying causes delayed pulmonic sound. Fixed splitting: Seen in ASD. ASD → left-to-right shunt → ↑ RA and RV volumes → ↑ flow through pulmonic valve. Pulmonic closure is greatly delayed. Regardless of inspiration. Paradoxical splitting: Seen in conditions that delay LV emptying (aortic stenosis, LBBB). During inspiration P2 moves closer to A2, thereby "paradoxically" eliminating the split. |
|
|
Ventricular action potential Also occurs in bundle of His and Purkinje fibers |
Phase 0 = rapid upstroke and depolarization (voltage-gated Na channels open) Phase 1 = Initial repolarization (Inactivation of voltage-gated Na channels. Voltage-gated K channels begin to open) Phase 2 = plateau (Ca influx balance K efflux. Ca influx triggers Ca release from sarcoplasmic reticulum and myocyte contraction) Phase 3 = rapid depolarization (massive K efflux due to opening of voltage-gated slow K channels and closure of voltage-gated Ca channels) Phase 4 = resting potential (high K permeability through K channels) |
|
|
Pacemaker action potential Occurs in SA and AV nodes |
Phase 0 = upstroke (opening of voltage-gated Ca channels. Slow conduction velocity that is used by the AV node to prolong the transmission from atria to ventricles) Phase 3 = inactivation of Ca channels and ↑ activation of K channels → ↑ K efflux. Phase 4 = slow diastolic depolarization (membrane potential spontaneously depolarizes as Na conductance ↑. Accounts for automaticity of SA and AV nodes. The slope of phase 4 in the SA node determines HR. |
|
|
Electrocardiogram P wave - atrial depolarization (normally < 100 msec) PR interval - conduction delay through AV node (normally < 200 msec) QRS complex - ventricular depolarization (normally < 120 msec) QT interval - mechanical contraction of ventricles T wave - ventricular repolarization ST segment - isoelectric, ventricles depolarized U wave - caused by hypokalemia, bradycardia |
Speed of conduction - Purkinje > atria > ventricles > AV node Pacemakers - SA > AV > bundle of His/Purkinje/ventricles Conduction pathway - SA → atria → AV → common bundle → bundle branches → Purkinje fibers → ventricles |
|
|
Torsades de pointes Polymorphic ventricular tachycardia. Shifting sinusoidal waveforms on ECG; can progress to ventricular fibrillation. Long QT interval predisposes to torsades de pointes. |
Some Risky Meds Can Prolong QT Sotalol Risperidone (antipsychotics) Macrolides Chloroquine Protease inhibitors (-navir) Quinidine (class Ia; also class III) Thiazides |
|
|
Congenital long QT syndrome |
Inherited disorder of myocardial repolarization, typically due to ion channel defects; ↑ risk of sudden cardiac death due to torsades de pointes. Includes: Romano-Ward syndrome: autosomal dominant, pure cardiac phenotype (no deafness) Jervell and Lange-Nielsen syndrome: autosomal recessive, sensorineural deafness |
|
|
Wolff-Parkinson-White syndrome |
Most common type of ventricular pre-excitation syndrome. Abnormal fast accessory conduction pathway from atria to ventricle bypasses the rate-slowing AV node. Ventricle begin to partially depolarize earlier, giving rise to characteristic delta wave with shortened PR interval on ECG. |
|
|
Atrial fibrillation Atrial flutter |
Atrial fibrillation Chaotic and erratic baseline. No P waves between irregularly spaced QRS complex. Can result in atrial stasis and lead to thromboembolic stroke. Treatment includes rate control, anticoagulation, and possible pharmacological and electrical cardioversion
Atrial flutter Rapid succession of identical, back-to-back atrial depolarization waves. Pharmacologic conversion to sinus rhythm: class IA, IC, or III antiarrhythmics. Rate control: β-blocker or calcium channel blocker. Definitive treatment: catheter ablation. |
|
|
Ventricular fibrillation AV block 1st degree |
Ventricular fibrillation Completely erratic rhythm with no identifiable waves. Fatal arrhythmia without immediate CPR and defibrillation.
AV block 1st degree The PR interval is prolonged (>200 msec). Benign and asymptomatic. No treatment required. |
|
|
AV block 2nd degree (Mobitz I [Wenckebach] and II) AV block 3rd degree (complete) |
AV block 2nd degree Mobitz type I (Wenckebach) Progressive lengthening of PR interval until a beat is dropped. Usually asymptomatic.
AV block 2nd degree Mobitz type II Dropped beats that are not preceded by a change in the length of PR interval. May progress to complete block. Treated with pacemakers.
AV block 3rd degree (complete) Both atria and ventricles beat independently of each other. P waves and QRS complex present but unrelated. Treated with pacemaker. Lyme disease can result in complete block. |
|
|
Atrial natriuretic peptide B-type (brain) natriuretic peptide |
Atrial natriuretic peptide Released from atrial myocytes in response to ↑ blood volume and atrial pressure. Causes vasodilation and ↓ Na reabsorption at renal collecting tubule. Promoted diuresis and contributes to "aldosterone escape" mechanism.
B-type (brain) natriuretic peptide Released from ventricular myocytes in response to ↑ tension. Similar physiologic action to ANP, with longer half life. BNP blood test used for diagnosing HF (very good negative predictive value). Available in recombinant form (nesiritide) for treatment of HF. |
|
|
Right-to-left shunts |
Early cyanosis - "blue babies". Diagnosed prenatally o evident immediately after birth. Usually require urgent surgical correction and/or maintenance of PDA The 5Ts: 1) Truncus arteriosus (1 vessel) 2) Transposition (2 switched vessels) 3) Tricuspid atresia (3 = Tri) 4) Tetralogy of Fallot (4 = Tetra) 5) TAPVR - Total anomalous pulmonary venous return (5 letters in name) |
|
|
Persistent truncus arteriosus D-transposition of great vessels Right-to-left shunts |
Persistent truncus arteriosus Failure of truncus arteriosus to divide into pulmonary trunk and aorta; most patients have accompanying ventricular septal defect.
D-transposition of great vessels Aorta leaves RV and pulmonary trunk leaves LV → separation of systemic and pulmonary circulations. Not compatible with life unless a shunt is present. Due to failure of the aorticopulmonary septum to spiral. Without surgical intervention, most infants die within the first few months of life. |
|
|
Tricuspid atresia Tetralogy of Fallot Total anomalous pulmonary venous return (TAPVR) Right-to-left-shunts |
Tricuspid atresia Absence of tricuspid valve and hypoplastic RV; requires both ASD and VSD for viability.
Tetralogy of Fallot Most common cause of early childhood cyanosis, caused by anterosuperior displacement of infundibular septum. PROVe: Pulmonary fundibular stenosis Right ventricular hypertrophy - boot shaped heart on CXR Overriding aorta Ventricular septal defect
TAPVR Pulmonary veins drain into right heart circulation; associated with ASD and sometimes patent ductus arteriosus to allow right-to-left shunting to maintain CO |
|
|
Left-to-right shunts Ventricular septal defect Atrial septal defect Patent ductus arteriosus Eisenmenger syndrome |
Late cyanosis - "blue kids"
Frequency VSD > ASD >PDA |
|
|
Ventricular septal defect Atrial septal defect Left-to-right shunts |
Ventricular septal defect Most common cardiac congenital defect. Asymptomatic at birth, may manifest weeks later or remain asymptomatic. Most self resolve; larger lesions may cause LV overload and HF.
Atrial septal defect Defect in interatrial septum; loud S1; wide, fixed split S2. Usually occurs in septum secundum. Symptoms range from none to HF. Distinct from patent foramen ovale in that septa are missing tissue rather than unfused. |
|
|
Patent ductus arteriosus Left-to-right shunt |
Patent ductus arteriosus In neonatal period, ↓ lung resistance → shunt becomes left-to-right → progressive RVH and/or LVH and HF. Associated with continuous murmur. Patency is maintained by PGE synthesis and low O2 tension. PDA can eventually result in late cyanosis in lower extremities (differential cyanosis) Indomethacin ("endomethacin") ends patency of PDA; PGE kEEps it open. PDA is normal in utero (right-to-left shunt) and normally closes only after birth. |
|
|
Eisenmenger syndrome Left-to-right shunt |
Eisenmenger syndrome Uncorrected left-to-right shunt → ↑ pulmonary blood flow → pathologic remodeling of vasculature → pulmonary arteriolar hypertension. RVH occurs to compensate → shunt becomes right-to-left. Causes late cyanosis, clubbing, and polycythemia. Age of onset veries |
|
|
Coarctation of the aorta Infantile type Adult type |
Coarctation of the aorta Associated with bicuspid aortic valve, other heart defects. Infantile type Aorta narrowing is proximal to insertion of ductus arteriosus. Associated with Turner syndrome. Can present with closure of ductus arteriosus. Infantile: in close to the heart. Adult type Aorta narrowing is distal to ligamentum arteriosum. Associated with notching of the ribs (collateral circulation), hypertension in upper extremities, and weak, delayed pulses in lower extremities (radiofemoral delay). Adult: distal to ductus. |
|
|
Congenital cardiac defect associations 22q11 syndromes Down syndrome Congenital rubella Turner syndrome Marfan syndrome Infant of diabetic mother |
22q11 syndromes - Truncus arteriosus, tetralogy of Fallot Down syndrome - ASD, VSD, AV septal defect Congenital rubella - Septal defects, PDA, pulmonary artery stenosis Turner syndrome - Bicuspid aortic valve, coarctation of aorta (preductal) Marfan syndrome - Mitral valve prolapse, thoracic aortic aneurysm and dissection, aortic regurgitation Infant of diabetic mother - Transposition of great vessels |
|
|
Hypertension Defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg |
Risk factors ↑ age, obesity, diabetes, smoking, genetics, black>white>asian. Features 90% of hypertension is essential (1°) and related to ↑ CO or ↑ TPR; remaining 10% mostly 2° to renal disease, including fibromuscular dysplasia in young patients. Hypertensive emergency - Severe hypertension (≥ 180/120) with evidence of acute, ongoing target organ damage. Predisposes to Atherosclerosis, LVH, stroke, CHF, renal failure, retinopathy, and aortic dissection. |
|
|
Hyperlipidemia signs |
Xanthomas - plaques or nodules composed of lipid-laden histiocytes in the skin, especially the eyelids (xanthelasma). Tendinous xanthoma - Lipid deposit in tendon, especially Achilles. Corneal arcus - Lipid deposit in the cornea, appears early in life with hypercholesterolemia. Common in elderly (arcus senilis). |
|
|
Arteriosclerosis Mönckeberg (medial calcific stenosis) Arteriolosclerosis |
Mönckeberg (medial calcific stenosis) Uncommon. Calcification in the media of the arteries, especially radial or ulnar. Usually benign; "pipestern" arteries on x-ray. Does not obstruct blood flow; intima not involved. Arteriolosclerosis Common. Two types: hyaline (thickening of small arteries in essential hypertension and DM) and hyperplastic ("onion skinning" as seen in severe hypertension). |

