![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
|
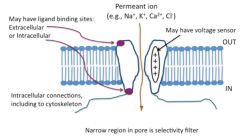
Describe a generic ion channel:
types of gating and sensors-- configurations-- |
|
|
|
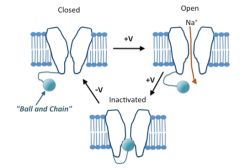
Describe configurations of ion channels:
|
|
|
|
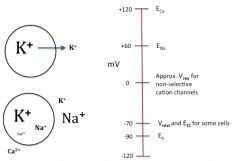
Ion concentrations and Nernst voltages for Na, K, Ca:
|
|
|
|
What is the Nernst potential?
|

|
|
|
What is an ion's reversal potential?
|

|
|
|
How are ion gradients maintained?
3 important pumps: |

|
|
|
How do cardiac glycosides work?
What's an important example of one? What does it do? |

|
|
|
How do diuretics affect the CV system?
What's an important example? Where and how does it act? |

|
|
|
Local potentials, action potentials and threshold:
|
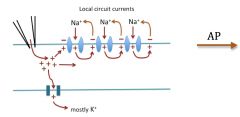
Action potentials are all or none. APs travel down the membrane. Gap junctions allow charge to flow to next membrane over and depolarize it. It's almost like the heart is one big cell.
|
|
|
How do local anesthetics affect action potentials?
What's an example? |
If many of the Na+ channels are blocked by local anesthetics or neurotoxins, or there is nerve damage, threshold will be elevated and action potentials will occur less frequently or not at all.
This happens to the pain-sensing neurons in your skin when you spray on a local anesthetic like Bactine. |
|
|
What effect can a local potential have on an action potential?
|
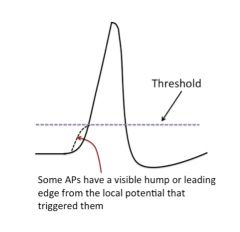
|
|
|
Ion channel activity underlying action potential:
|
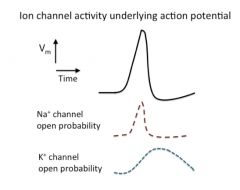
|
|
|
Discuss refractory periods:
|
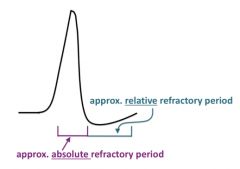
*Na+ channel inactivation limits the frequency of repetitive stimulation, since new action potentials cannot occur if too many of the Na+ channels are inactivated, and therefore are not able to open.
*The period of Na+ channel inactivation, when it is impossible to evoke another action potential, is called the "absolute refractory period"; this prevents the overlap or summing of action potentials, leaving each impulse a distinct signal like a dot in a Morse code. *It is the frequency of action potentials that is used to encode information and commands in the nervous system. |
|
|
Discuss accommodation:
|
If a membrane is slowly or slightly, but continuously, depolarized (a few millivolts may be enough), the available Na+ channels will progressively become inactivated, resulting in "accommodation".
In this state, action potential frequency diminishes, and in extreme cases, spikes cannot be elicited no matter how much depolarizing input occurs. This phenomenon can occur with ischemia of the heart as well as with many other pathophysiological conditions. |
|
|
What is the relative refractory period?
|
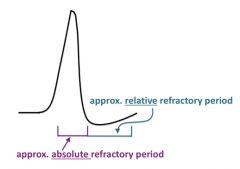
During the "relative refractory period" the Na+ channels are no longer inactivated, but the K+ channels are still open, keeping the potential near the K+ Nernst potential.
Thus, it is harder to elicit an action potential because the membrane potential is farther from the depolarized level (threshold) at which enough Na+ channels can be opened to start the spike. The relative refractory period is a bit different in the heart (see below). |
|
|
Discuss hyperkalemia:
|
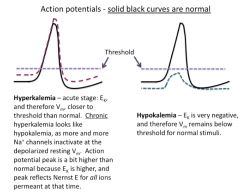
Clinically, an elevation in extracellular K+ ("hyperkalemia", as may occur during kidney failure) produces a depolarization that initially increases the "excitability" of nerve and muscle cells (i.e., makes them fire action potentials more easily), and then, if sustained, can lead to accommodation.
Thus, a person with elevated extracellular K+ may have spontaneous muscle twitches, seizures or even asphyxiation due to spastic contraction of the respiratory muscles. If the person survives the increased excitation, he or she may then enter a state of muscle weakness or paralysis (along with other symptoms) because of low excitability resulting from accommodation. |
|
|
Discuss hypokalemia:
|
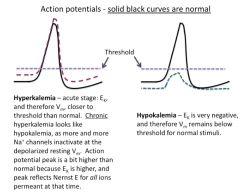
Interestingly, the symptoms from prolonged elevated K+ resemble those of acutely decreased extracellular K+ (hypokalemia), which gives a hyperpolarization that depresses excitability of nerve and muscle.
Both hypokalemia and hyperkalemia can lead to cardiac arrhythmias and other life-threatening phenomena. Why does an increase in extracellular K+ produce a depolarization, and a decrease produce a hyperpolarization? Hint: think about the K+ Nernst potential and its effect on membrane potential. |
|
|
Discuss hypocalcemia:
|
Changes in extracellular Ca2+ (as can occur, for example, with parathyroid or kidney problems) can cause clinically relevant changes in the threshold for action potential generation.
Under normal conditions, extracellular Ca2+ associates with negative charges on the surface of the membrane (on lipids, proteins and sugar residues), and also with binding sites on ion channels, inhibiting the opening of many ion channels (including voltage-gated Na+ channels). When extracellular Ca2+ falls below normal (hypocalcemia), some Ca2+ leaves the channels and the membrane surface, relieving channel inhibition and thereby reducing the threshold for Na+ channel activation, so that Na+ channels open at less positive voltages than are normally required. Thus, the neurons and muscle cells become too excitable. This hyperexcitability can result in hyper-reflexia, spontaneous twitching, muscle cramps, tingling, and in extreme cases, "low-calcium tetany" -- a condition in which the muscles display an abnormal sustained contraction. |
|
|
Discuss hypercalcemia:
|
When extracellular Ca2+ is elevated (hypercalcemia), the opposite occurs: excitability of neurons and muscle cells is depressed, giving such symptoms as muscle weakness, paralysis and coma.
Both increases and decreases in extracellular Ca2+ can cause dangerous cardiac arrhythmias, as well as problems in the central nervous system, such as memory loss and confusion, and both are life-threatening. However, the changes in extracellular Ca2+ needed to elicit these effects do not significantly change the cell's membrane potential -- instead, they appear to change how the ion channels sense and respond to the membrane potential. This mechanism is different from that with changes in extracellular K+. Because of the common symptoms of hypokalemia, chronic hyperkalemia and hypercalcemia, blood work is especially important in discriminating the underlying conditions. |
|
|
Schematic of electrical conduction in the heart:
|
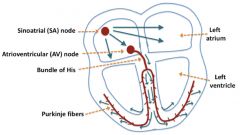
|
|
|
Describe the process of electrical conduction in the heart:
|
The pumping of the heart is triggered by an orderly electrical excitation that spreads throughout the heart, stimulating the muscles to contract. This excitation occurs in specialized heart muscle cells called conducting cells (as compared with the contracting cells that do the mechanical pumping of blood).
The excitation normally begins in the sinoatrial (SA) node near the top of the right atrium, then spreads out over the right and left atria and simultaneously to the atrioventricular (AV) node. From this point, the excitation spreads into the conducting cells of the ventricles: down the common bundle to the bundle of His, to the right and left bundle branches, and then to the Purkinje fibers. From here, the action potential spreads up the ventricles to stimulate ejection of the blood. Spread is via gap junctions (intercalated disks). Note that it is really ions (mostly K+, since it is most abundant) that travel through the gap junction channels, not the action potentials per se, so that in some cases the form of the action potential can be very different in the pre- and post-synaptic cells. For example, in the heart, since SA node cells are connected to atrial muscle cells by gap junctions, the action potential in the SA node cell is a Ca2+ spike with an underlying pacemaker potential, and the action potential in the atrial muscle is a typical broad cardiac muscle action potential with a Na+ spike and a Ca2+/K+ plateau. |
|
|
Discuss different conduction speeds in the heart:
What determines where the pacemaker is? |
The slowest conduction (spread) of the action potential is in the AV node, where the rate is only 0.01 to 0.05 m/sec. This slow rate produces the AV delay that is required to insure that the ventricles do not contract before they have had time to fill with blood from the atria.
Conduction in the atria and ventricles is about 1 m/sec, and the rate in the bundle of His and Purkinje fibers is 2 to 4 m/sec to promote rapid activation of the ventricles for efficient ejection of blood. Interestingly, the conduction rate in the SA node is similar to that in the AV node, but the intrinsic pacemaker frequency in the SA node cells is faster than that in the AV node cells (e.g., 60 vs. 40 spikes per minute, respectively), so that the SA node normally sets the heart rate. The timing and pattern of the spread of excitation is critical. When it is normal, following the route described above, with a rate of 60 to 100 beats per minute, it is called normal sinus rhythm. |
|
|
Discuss the cardiac AP:
|
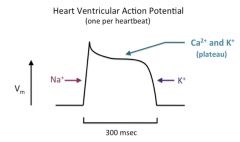
The action potentials of ventricular and atrial heart muscle cells begin with a Na+ spike (phase 0), followed by a brief repolarization (phase 1) due to Na+ channel inactivation and the opening of very slow delayed rectifier K+ channels, and then a long plateau phase (phase 2) that results from the opening of voltage-gated Ca2+ channels (mostly L-type, but some T-type channels), and at the same time, several other types of K+ channels.
Calcium entry through the voltage-gated Ca2+ channels triggers calcium-induced calcium release from the cardiac SR, and is used in contraction (to be discussed in a later lecture). The plateau results from the balance between outward K+ current and inward Ca2+ current, and is longer in ventricular action potentials than in those from the atrium. In each case, the plateau is followed by a repolarization (phase 3) to the resting potential (phase 4) as a result of continued activity of K+ channels. Heart muscle cells (ventricular and atrial) have a more negative resting potential (around -85 or -90 mV) than most cells, since they have so many open K+ channels that drive the membrane potential toward the K+ Nernst potential and away from threshold when the cell is between action potentials. Thus, ventricular and atrial action potentials don't have an undershoot at the end, since their resting Vm is already so close to Ek. |
|
|
Discuss the automaticity of the heart:
|
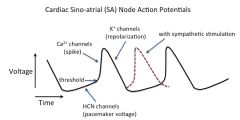
The heartbeat is normally set by a spontaneous rhythmic fluctuation (automaticity) in the membrane voltage of specialized muscle cells in the sinoatrial (SA) node region of the heart. This voltage fluctuation is called the "pacemaker potential" and results from the opening and closing of several kinds of ion channels.
The ion channel that appears to be crucial for the slow positive swing in membrane voltage leading to the upstroke of the action potential is the nonselective cation channel that produces an inward current called Ih (HCN)(or If). This channel is opened by negative voltages, and its voltage sensitivity is regulated by the direct binding of cyclic adenosine monophosphate (cAMP; see autonomic control section). Note that this action potential does not have a significant plateau phase or an early repolarization phase; thus, it has only phases 0, 3 and 4 (see Costanzo, Figure 4-12). Furthermore, phase 4 is not a constant resting potential as in ventricular and atrial action potentials; instead, it consists of a slow depolarization from its most negative value (around -65 mV), the maximum diastolic potential. |
|
|
What's the current theory on how the pacemaker potential works?
|
a. The cell membrane potential starts out negative b/c of a large number of open K+ channels.
b. This membrane hyperpolarization induces the opening of nonselective cation channels (“pacemaker” channels, Ih or If), resulting mainly in a net influx of Na+, which, in turn, causes the membrane to depolarize to threshold. [Note: unfortunately, some textbooks refer to these channels as Na+ channels, which can be confusing – they are nonselective cation channels, not voltage-gated Na+ channels; because of the gradients, Na+ is the main ion carrying current through these channels, into the cells, giving a depolarization.] c. The depolarization opens Ca2+-selective ion channels, both "T-type" and "L-type". The T- type channels open near the end of phase 4, and the L-type channels open during phase 0, producing the Ca2+ spike. [The "T-type" and "L-type" designations refer to the different gating kinetics of the two classes of Ca2+ channels: "T-type" channels are more transient, i.e., have faster inactivation kinetics, whereas "L-type" have slower inactivation kinetics, i.e., are open longer] d. This depolarization causes a delayed opening of additional K+ channels ("delayed rectifier" channels), sending the membrane potential negative again, thereby causing closure of the voltage-gated Ca2+ channels, which leads to further hyperpolarization. e. This hyperpolarization reactivates the nonselective cation channels (Ih channels), and deactivates the delayed rectifier K+ channels, starting the cycle over again. |
|
|
Discuss the latent pacemakers of the heart:
|
The atrioventricular (AV) node, bundle of His and Purkinje fibers are latent pacemakers of the heart. However, normally the pacemaking activity of the SA node is faster, and therefore dominates, giving a phenomenon called “overdrive suppression.”
A latent pacemaker can take over, as an ectopic pacemaker or ectopic focus, if the pacemaking activity of the SA node becomes slower than that of the latent pacemaker, or if conduction of the action potential from the SA node to the atria and ventricles is blocked. These events can occur with vagal stimulation (see below), heart damage or drugs. Obviously, normal heart function will be disrupted if there is activity of competing pacemakers with similar firing rates, but in different locations. |
|
|
How is the Purkinje AP different than ventricular or atrial AP?
|
![Purkinje fibers have action potentials that are similar to those of the ventricles and atria, including having a Na+ spike [not a Ca2+ spike] and having a long plateau phase. However, the baseline of a Purkinje fiber action potential is not flat; ...](https://images.cram.com/images/upload-flashcards/52/73/78/2527378_m.jpg)
Purkinje fibers have action potentials that are similar to those of the ventricles and atria, including having a Na+ spike [not a Ca2+ spike] and having a long plateau phase. However, the baseline of a Purkinje fiber action potential is not flat; instead, it has a slight upward trend toward threshold (but this spontaneous depolarization is much less pronounced than that of the SA node cells).
On the other hand, action potentials in the AV node are Ca2+ spikes that more closely resemble those of the SA node. Note phases of the cardiac AP. Be able to draw and label this. |
|
|
Discuss cardiac AP absolute and relative refractory periods:
|
Absolute (Effective): cannot generate an action potential no matter how great the stimulus, i.e., transient inexcitability after depolarization. This occurs because too many voltage-gated channels are inactivated (these are Na+ channels in the ventricular and atrial muscle cells and Purkinje fibers, but Ca2+ channels in the SA and AV nodes). For the ventricular AP, the absolute refractory period would be from phase 0 through the end of phase 2. For the SA node cell, this would be from phase 0 through most of phase 3.
Relative: can generate an action potential, but must use a stimulus that is greater than normal, since not all the voltage-gated ion channels have recovered from inactivation. This occurs during the repolarization phase (phase 3). |
|
|
Discuss sympathetic control of the heart:
|
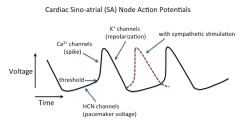
The sympathetic nervous system speeds the heart, and the parasympathetic nervous system slows the heart.
The binding of cAMP directly to an intracellular site on the pacemaker (Ih) channel causes the channel to open more quickly and at relatively less negative voltages than if cAMP were not bound. Thus, in the presence of cAMP, the positive swing in the pacemaker potential (phase 4) occurs sooner and is more steep, and consequently the upstroke of the action potential occurs sooner, leading to a higher frequency of action potentials and therefore a faster heartbeat. [In the current scientific literature, the pacemaker is called the HCN channel, for Hyperpolarization-activated, Cyclic Nucleotide-regulated channel.] This is, in part, how epinephrine speeds the heart -- i.e., by activating beta-adrenergic (β1) receptors, which stimulate adenylyl cyclase via G-proteins, increasing [cAMP], and thereby increasing the frequency of SA node action potentials. The epinephrine-induced increase in [cAMP] also stimulates cAMP-dependent protein kinase, which phosphorylates Ca2+ channels, making it easier for them to open as well. “Beta-blocker” drugs (β1 antagonists) block these effects, slowing the heart, reducing blood pressure. The above mechanism is called positive chromotropic effects. The autonomic system also affects action potential conduction velocity (dromotropic effects) by controlling the amount of inward and outward current, and therefore the rate at which threshold is reached. Sympathetic stimulation increases inward Ca2+ current. |
|
|
Discuss PS effects on the heart:
|
Parasympathetic nerves (e.g., vagus) release acetylcholine (ACh), which activates muscarinic (M2) receptors in the SA node. These receptors in turn activate an inhibitory G-protein (GK) that has two effects on the system: it inhibits adenylyl cyclase, decreasing cAMP; and it directly activates K+-ACh channels, hyperpolarizing the cell to keep it below threshold.
The above mechanism is called negative chromotropic effects. The autonomic system also affects action potential conduction velocity (dromotropic effects) by controlling the amount of inward and outward current, and therefore the rate at which threshold is reached. Parasympathetic stimulation decreases inward Ca2+ current and increases outward K+-ACh current. |

