![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
58 Cards in this Set
- Front
- Back
|
Monosaccharide |
The simplest form of carbohydrates. They consist of one sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose (dextrose), fructose, galactose, and ribose. |
|
|
Trioses |
Contain 3 Carbons |
|
|
Tetroses |
Contain 4 Carbons |
|
|
Pentoses |
Contain 5 Carbons |
|
|
Hexoses |
Contain 6 Carbons |
|
|
Aldoses |
Contain an Aldehyde functional group as the most oxidized functional group H-C=O Ex. Glucose |
|
|
Ketoses |
Contain a Ketone functional group as the most oxidized functional group R-C(=O)-R Ex. Fructose |
|
|
Glyceraldehyde (3 carbon monosaccharide with an aldehyde) |
Aldehyde = Aldo 3 Carbon = Triose Aldo+Triose = Aldotriose |
|
|
Dihydroxyacetone (3 carbon monosaccharide with a ketone) |
Ketone = Keto 3 Carbon = Triose Keto+Hexose = Ketotriose |
|
|
D vs L Orientation |
For sugars with more than one chiral center, D or L designates the asymmetric carbon farthest from the aldehyde or keto group. Hydroxyl (OH) group on the last chiral carbon = right side, D enantiomer. Hydroxyl on the last chiral carbon = left side, L enantiomer. Most naturally occurring sugars are D isomers. |
|
|
D-Fructose |
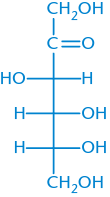
|
|
|
D-Glucose |
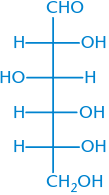
|
|
|
D-Galactose |
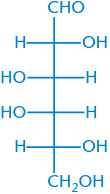
|
|
|
D-Mannose |
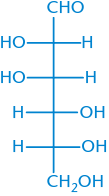
|
|
|
Enantiomers |
Same sugar (molecule), different optical family Ex. D-Glucose vs. L-Glucose |
|
|
Diastereomers |
Two sugars in the same family (both are either Ketoses or Aldoses and have the same number of carbons), but are not identical and are not mirror images. |
|
|
Epimers |
A special subtype of diastereomers. Molecules that differ in configuration at exactly one chiral center. |
|
|
Hemiacetal |
The product of adding an Alcohol (R-O-H) to a Carbonyl (Aldehyde, R-C(=O)-H) is known as a __________. |
|
|
Hemiketal |
The product of adding an Alcohol (R-O-H) to a Carbonyl (Ketone, R-C(=O)-R) is known as a __________. |
|
|
Hemiacetals (Cyclic) |
Monosaccharides contain both a hydroxyl (electrophile) and a carbonyl (nucleophile). Aldoses can undergo intermolecular reactions to form Cyclic Hemiacetals. |
|
|
Hemiketals (Cyclic) |
Monosaccharides contain both a hydroxyl (electrophile) and a carbonyl (nucleophile). Ketoses can undergo intermolecular reactions to form Cyclic Hemiketals. |
|
|
Pyranose Rings |
6-membered monosaccharide rings. |
|
|
Furanose Rings |
5-membered monosaccharide rings. |
|
|
Anomeric Carbon |
The Hydroxyl group acts as a nucleophile during ring formation, causing the Oxygen to become a member of the ring structure. During this process, the Carbonyl becomes chiral, and is known as the Anomeric Carbon (C-1). |
|
|
α-Anomer |
The Hydroxyl (-OH) group on C-1 is Trans to the -CH2OH substituent. |
|
|
β-Anomer |
The Hydroxyl (-OH) group on C-1 is Cis to the -CH2OH substituent. |
|
|
Mutarotation |
Exposing Hemiacetal rings to water causes them to spontaneously cycle between the open/closed forms. This in turn causes the ability for both α & β anomers to form due to rotation on the single bond between C-1 and C-2. -The spontaneous change in configuration is known as Mutarotation. |
|
|
Aldonic Acids |
Monosaccharide (aldehyde) in open chain form becomes oxidized, creating __________ _________. |
|
|
Reducing Sugar |
Any monosaccharide with a hemiacetal ring. |
|
|
Lactone |
An Aldose in ring form (Hemiacetal) undergoes oxidation to yield a ________. This is a cyclic ester with a carbonyl group on the anomeric carbon. Carbonyl group: R-C(=O)-R |
|
|
Tollen's Reagent |
Utilizes Ag(NH3)2, Silver Nitrate, as an oxidizing agent. -Positive: Aldehydes reduce Ag+ to metallic silver. |
|
|
Benedict's Reagent |
Oxidizes the Aldose group of an Aldehyde, and is indicated by the precipitate of Cu2O, which is Red. |
|
|
Keto-Enol Shift |
The rearrangement of a compound, usually by moving a hydrogen ion and forming a double bond (Tautomerization). In this case, the ketone group picks up a hydrogen and the double bond is moved between two adjacent carbons, resulting in an enol (compound with a double bond and an alcohol group). |
|
|
Alditol |
When the Aldehyde group of a sugar is reduced to an alcohol, the compound is considered an __________. |
|
|
Deoxy Sugar |
Contains a Hydrogen that replaces a Hydroxyl group (H insteand of OH) on the sugar. The most well known of these sugars is D-2-Deoxyribose, the carbohydrate found in DNA. |
|
|
Phosphorylation |
A type of esterification reaction that includes a Nucleophillic attack by a Hydroxyl (OH) group on a Phosphate (PO4) group from ATP, reducing it to ADP and transferring the phosphate group. -Nucleophile: OH -Electrophile: PO4 |
|
|
Acetals |
Hemiacetals react with alcohols to form _______. The anomeric hydroxyl group is transformed into an alkoxy group (RO), yeilding a mixture of α & β acetals, with water as a leaving group. The resulting C-O bonds are Glycosidic bonds, and the acetals formed are Glycosides. |
|
|
Ketals |
Hemiketals react with alcohols to form _______. The anomeric hydroxyl group is transformed into an alkoxy group (RO), yeilding a mixture of α & β ketals, with water as a leaving group. The resulting C-O bonds are Glycosidic bonds, and the ketals formed are Glycosides. |
|
|
Glycoside |
A molecule that contains a bond between a functional group and a carbon of the sugar. Glycoside formation is a dehydration reaction, so breaking a Glycosidic bond requires hydrolysis. |
|
|
Glycosidic Bond |
The bond that forms between a functional group and a carbon of the sugar. This is the same type of bond that attaches monosaccharides together to create disaccharides. |
|
|
Furanosides |
Glycosides derived from Furanose Rings are referred to as ___________. |
|
|
Pyranosides |
Glycosides derived from Pyranose Rings are referred to as ____________. |
|
|
Disaccharides |
A Glycosidic bond forms between hydroxyl groups of two monosaccharides, resulting the the expulsion of H2O and the creation of a Disaccharide. The Hydroxyl on the anomeric carbon reacts with the hydroxyl on another sugar to form an acetal/ketal with a 1,2; 1,4; or 1,6 glycosidic link. |
|
|
α & β Glycosidic Linkage |
α or β Anomeric carbon forms a bond with a hydroxyl group on another sugar (of either α or β). -Ex. α-1,6 between two D-Glucose molecules. The bond is between the OH on the Anomeric carbon on G1, and the 6th carbon on G6. -Ex. β,β-1,1 bond is between the OH on both Anomeric carbons. |
|
|
Sucrose |
glucose-α-1,2-fructose |
|
|
Lactose |
galactose-β-1,4-glucose |
|
|
Maltose |
glucose-α-1,4-glucose |
|
|
Polysaccharides |
Long chains of monosaccharides linked together by glycosidic bonds. |
|
|
Homopolysaccharides |
A Polysaccharide comprised of one type of monosaccharide (ex. all glucose). |
|
|
Heteropolysaccharides |
A Polysaccharide comprised of more than one type of monosaccharide (ex. glucose and fructose). |
|
|
Cellulose |
Main structural component of plants. Comprised of β-D-glucose molecules linked byβ-1,4 glycosidic bonds. -Homopolysaccharide. -Requires cellulase to digest. |
|
|
Starches |
Polysaccharides that are more digestible by humans due to linked α-D-glucose monomers. |
|
|
Amylopectin |
Starch comprised of α-D-glucose molecules linked by α-1,4 glycosidic bonds, with branches via α-1,6 glycosidic bonds (approx. 1/25 glucose molecules) Debranching enzymes help to degrade the polysaccharide. |
|
|
Amylose |
Plants mainly store starch as Amylose, comprised of α-D-glucose molecules linked by α-1,4 glycosidic bonds. -Broken down by α or β amylase. Iodine tests for the presence of starch by fitting inside the helix conformation made by amylose, creating a starch-iodine complex. |
|
|
β Amylase |
Cleaves Amylose at non-reducing end (the end with the acetal), yielding Maltose. |
|
|
α Amylase |
Cleaves Amylose randomly along the chain to create shorter polysaccharide chains, maltose, and glucose. |
|
|
Glycogen |
Main carbohydrate storage in animals. Similar structure to starch, but includes α-1,6 glycosidic bonds (approx. 1/10 glucose molecules) which yields a highly branched product. Branching optimizes energy efficiency, increases solubility in solution, and allows for enzymes to work on multiple sites simultaneously. |
|
|
Glycogen Phosphorylase |
Functions by cleaving glucose from the nonreducing end of a glycogen branch and phosphorylating it, producing glucose 1-phosphate. Glucose 1-phosphate plays an important role in metabolism. |

