![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
How to work out molar mass? |

Sum up relative atomic mass of every atom in formula |
|
|
Metal carbonate will thermally decompose to what? |
Metal oxide + carbon dioxide |
|
|
How to work out number of moles when given mass of the chemical? (Equation ) |
Number of moles = mass of chemical / molar mass |
|
|
Mole is?? |
One product. E.g. MgO is one mole. MgO is 40g (molar mass) 0.5 mole of it has 20g |
|
|
Empirical formula |

|
|
|
Empirical formulae from actual mass |

|
|
|
Formula for finding the percentage of an element in a compound? |
% mass of element = total mass of the element in the compound / relative formula mass of compound * 100 |
|
|
As concentration increases, the solute particles become...? |
More crowded |
|
|
Remember |

|
|
|
To convert cm^3 into dm^3... To convert dm^3 to cm^3... |

|
|
|
How to work out amount in moles with concentration and volume? |
Amount in moles = concentration * volume |
|
|
Tip |
The government has introduced a colour coded " traffic light " system for food labels showing the types of food such as sugar, salt and fat and their quantities. The label also gives the percentage of the guideline daily amounts, DGA, of these foods |
|
|
Indicator, litmus, gives what colour off for alkali? |
Blue |
|
|
Indicator litmus gives what colour in acid? |
Red |
|
|
Indicator, phenolphthalein, gives what colour in alkali? |
Pink |
|
|
Indicator, phenolphthalein, gives what colour in acid? |
Colourless |
|
|
Indicator, screened methyl orange, give what colour in alkali? |
Green |
|
|
Indicator, screened methyl orange, give what colour in acid? |
Pink |
|
|
What are the three ways to collect a gas given off in a reaction so that the gas volume can be measured? |
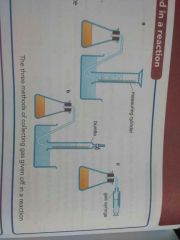
With... * measuring cylinder * burette * gas syringe |
|
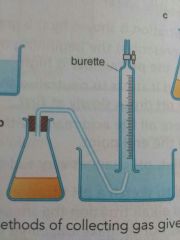
How can you measure how much gas volume was given off in a reaction using burette method? |

|
|
|
At room temperature and pressure, 1 mole of a gas takes up how much space (volume)? |
24 dm^3 |
|
|
Tip |

|
|
|
Reaction is at equilibrium when...? |
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. |
|
|
Equilibrium is always on what side of the equation? |
The side that has greater concentration of reactants/products |
|
|
If the concentration of reactants is greater than that of the products. The equilibrium position is... |
Left |
|
|
If the concentration of the reactants is less than that of the products, the equilibrium position is... |
Right |
|
|
Equilibrium only works if...? |
It is a closed system - The chemicals don't "get out" |
|
|
Remember |
Equilibrium only works if it is a closed system - the chemicals don't "get out" * initially, the forward reaction rate is fast, but then it slows as reactants are used up * as the same time, the backward reaction rate increases as more is available to react * eventually the backward reaction is as fast as the forward reaction. Equilibrium had been reached. |
|
|
What three things can alter equilibriums position? |
Temp, pressure and concentration |
|
|
Acids such as HCl contain hydrogen atoms. In water, what happens to the acids molecules? |
They ionise |
|
|
Strong acids ionise how much when they're in water? |
They ionise completely |
|
|
When there are lots of hydrogen ions in acid...? |
The acid is very acidic |
|
|
During hydrogen ions from acid reacting with water, what happens? |
Lots of collisions occur so the acid and water react quickly |
|
|
In a weak acids such as ethanoic acid , how much of it gets ionised in water? |
Very few acid molecules ionise |
|
|
In weak acid, isn't that acidic because...? |
It has less hydrogen ions |
|
|
During reaction with water, what happens to the reactants?( weak acid and water ) |
Fewer collisions occur so slower reaction |
|
|
High concentration in acid/alkali means... |
Low Ph number |
|
|
Low concentration of hydrogen in solution means...? |
High pH number |
|
|
Why weaker acids don't conduct as well as strong acids in electrolysis? |
Because they have less hydrogen ions to move via the liquid.
But the same volume of gas is made |
|
|
All acids produce H+ so in electrolysis, H+ (positive ions) will migrate to what? |
Cathode |
|
|
Stages of preparing a clean and dry sample of an insoluble chloride. |

|
|
|
What spectator ions? |
Ions that don't take part in the reaction |

