![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
383 Cards in this Set
- Front
- Back
|
what is the value of the pleural pressure during normal respiration?
|
negative
|
|
|
which bronchus is more inline with the trachea and is therefore more susceptible to aspiration bodies?
|
Right main bronchus
|
|
|
what type of epithelium is found in the bronchus?
|
pseudostratified, ciliated epithelium
|
|
|
what type of epithelium is found in the bronchiole?
|
simple columnar, ciliated
|
|
|
define deadspace
|
the area in the respiratory system where there is no gas exchange
|
|
|
give an example of a disease in which there is increased physiological deadspace
|
emphysema
|
|
|
which type of alveolar cells secrete surfactant?
|
Type II alveolar cells
|
|
|
major component of surfactant?
|
phospholipids
|
|
|
function of surfactant?
|
decreases surface tension, thereby preventing alveolar collapse
(also decreases intra-alveolar fluid accumulations) |
|
|
at what point in respiration is the resistance of the lung the least?
|
when the lung is fully inflated (at the end of inspiration)
|
|
|
what happens to resistance as lung volume decreases (expiration)?
|
resistance becomes greater
|
|
|
give an example of a lung disease that is significantly effected by dynamic closure.
|
emphysema
(high pressures move equilibratory point down to an area where the airway is collapsible. collapsible airways cause wheezing (or even collapse) upon respiration. |
|
|
at which week of gestation does formation of the respiratory tract begin?
|
week four
|
|
|
when does alveolar development:
1. begin 2. complete |
1. 34 weeks gestation
2. two years of age |
|
|
what are Clara cells?
|
non-ciliated cells that accumulate and detoxify some agents
|
|
|
what are Kulchitsky cells?
|
neuroedocrine cells
*secrete vasoactive amines and hormonally active polypeptides* |
|
|
chronic irritation causes the epithelium of the lung to change from ? to ?
|
columnar (simple or pseudostratified) to squamous
|
|
|
what is the Reid index?
|
ratio of the thickness of the mucous glands to the thickness of the bronchial wall.
|
|
|
what is an example of when the Reid index would be increased?
|
chronic bronchitis
|
|
|
T/F: cartilage and mucous secreting glands are found in the BRONCHIOLES
|
False
|
|
|
what cells are found in the bronchioles?
*significance? |
non-ciliated Clara cells
* clearance of airborne particles is difficult in the bronchioles due to the lack of ciliated cells* |
|
|
the respiratory unit of the lung is known as ?
|
an acinus
|
|
|
what lung components make up an acinus?
|
respiratory bronchioles
alveolar ducts alveoli |
|
|
what type of membrane is found between the alveoli?
|
fused basement membrane
|
|
|
a congenital abnormality of the lung in which incomplete or defective development occurs is called?
|
pulmonary hypoplasia
|
|
|
what is an example of compression of the lung that results in pulmonary hypoplasia?
|
diaphragmatic hernia
|
|
|
what is another important cause of pulmonary hypoplasia?
|
oligohydramnios
|
|
|
what is the difference between intra- and extra- lobular sequestration?
|
1. intralobular occurs within the visceral plerua
2. extralobular occurs outside of the visceral pleura |
|
|
diseases that present with hoarsness or voice changes are most likely diseases of?
|
the larynx
|
|
|
which type of reactive lesion of the larynx is caused by HPV?
|
squamous papilloma
rarely progresses to cancer |
|
|
95% of laryngeal carcinomas are of what type?
|
squamous cell carcinomas
|
|
|
most squamous cell carcinomas of the larynx are caused by?
|
irritation (esp. tobacco smoke)
|
|
|
infections seen in the bronchi and bronchioles are most common in what age group?
|
children
|
|
|
what is the significance of a smoker who inhales irritant gases?
|
many are oxidants and compound the chronic exposure to tobacco smoke
|
|
|
effects of ozone on airways?
|
makes airways more reactive and exacerbates asthma and other lung diseases
|
|
|
which irritant gas is found in industrial environments or decaying grain?
|
nitrogen dioxide
|
|
|
why is carbon monoxide so lethal?
|
binds hemoglobin, preventing oxygen biding and transport
|
|
|
inert matter (that may be smaller than vision allows) can cause diseases of the bronchi and bronchioles. what is another name for this matter?
|
particulate matter
|
|
|
a nonspecific granulomatous response in the bronchial tree is known as?
|
bronchocentric granulomatosis
|
|
|
what organism is known to cause bronchocentric granulomatosis in asthmatic patients?
|
Aspergillus
|
|
|
constrictive bronchiolitis involves constriction from?
|
submucosal thickening of bronchiolar wall
|
|
|
constrictive bronchiolitis will produce what pattern of lung disease?
|
obstructive pattern
|
|
|
what is bronchiectasis?
|
abnormal dilatation of the bronchi
|
|
|
from what do most lung tumors arise?
|
bronchial epithelium
|
|
|
what happens after prolonged atelectasis?
|
fibrotic scarring of the lung occurs
|
|
|
describe what happens in resorptive/obstructive atelectasis
|
*caused by an obstruction or mucous plug of bronchus
*residual air is resorbed and the lung collapses distal to the obstruction |
|
|
when the pleural cavity is filled with fluid or air, what type of atelectasis may occur?
|
compression atelectasis
|
|
|
which type of atelectasis is generally irreversible and what causes it?
|
contraction atelectasis
*occurs when scarring and contraction of the pleura prevents lung expansion |
|
|
fluid within the alveoli is known as?
|
pulmonary edema
|
|
|
what type of fluid is usually in the alveoli in pulmonary edema?
|
transudate
|
|
|
what am I called?
rapid onset of severe, life threatning respiratory insufficiency, tachy, cyanosis, severe arterial hypoxemia that is refractory to oxygen therapy? |
ARDS
adult respiratory distress syndrome |
|
|
mortality rate of ARDS?
|
~60%
|
|
|
pathogenesis of ARDS?
|
1. initial lesion is damage to alveolar wall
2. followed by vascular leakage, cell sloughing, edema, hyaline membrane formation, atelectasis 3. PROTEINACEOUS fluid accumulation is important in pathogenesis 4. compliment and cytokines play critical roles 5. Type II cell proliferation and interstitial inflammation, organization, fibrosis 6. fibrosis may resolve or death may result |
|
|
what is the most common cause of ARDS?
|
shock/sepsis
|
|
|
respiratory distress syndrome in infants is called?
|
hyaline membrane syndrome
|
|
|
what defines the two stages of ARDS?
|
1. acute stage, cellular
2. organization stage, fibrosis |
|
|
how long after the onset of the acute stage does the organization stage of ARDS begin?
|
~1wk
|
|
|
obstructive lung disease is caused by?
|
increased resistance to airflow from partial or complete obstruction at any level.
|
|
|
restrictive lung disease is caused by?
|
reduced capacity of lung due to decreased expansion of parenchyma
|
|
|
which disease type is known as a volume/capacity disease?
|
restrictive lung disease
|
|
|
which disease is known as an airway disease?
|
obstructive lung disease
|
|
|
chronic bronchitis is what type of lung disease?
|
obstructive
|
|
|
asthma is what type of lung disease?
|
obstructive
|
|
|
ARDS is what type of lung disease?
|
restrictive
(hyaline alveolar membranes) |
|
|
bronchiolitis is what type of lung disease?
|
obstructive
|
|
|
pneumoconiosus is what type of lung disease?
|
restrictive
|
|
|
interstitial/infiltrative lung disease is what type of lung disease?
|
restrictive
|
|
|
bronchiectasis is what type of lung disease?
|
obstructive
|
|
|
what is the major clinical symptom seen in obstructive lung disease?
|
dyspnea
|
|
|
what type of "chest" is seen in a COPD patient?
|
barrel chest
|
|
|
what are the three major components of COPD?
|
1. emphysema
2. chronic bronchitis 3. small airway obstruction |
|
|
what difference in the PFT would you see in a person with COPD?
|
decrease in FEV
|
|
|
what is panacinar emphysema?
|
emphysema involving the entire acinus from the respiratory bronchioles to the terminal acinus
|
|
|
describe centrolobular emphysema
|
emphysema involving the respiratory bronchiole only
(the terminal acinus is normal) |
|
|
where in the lung is centrolobular emphysema the worst?
|
upper lobes
apical segments |
|
|
where is paraseptal emphysema located?
|
along the pleura and perilobular space
|
|
|
what is bullous emphysema?
|
any form of emphysema resulting in air spaces over 1cm.
|
|
|
describe senile emphysema
|
larger ducts and smaller acini; seen with aging; elasticity is normal
|
|
|
which type of emphysema is seen in a-1-antitrypsin deficiency?
|
panacinar
|
|
|
which type of emphysema is most common in smokers with chronic bronchitis?
|
centrolobular
|
|
|
which organ makes alpha-1-antitrypsin?
|
liver
|
|
|
function of a-1-antitrypsin?
|
protects alveoli from destruction by inflammatory cell proteinases (trypsin, elastase)
|
|
|
what is a major source of elastase seen in the pathogenesis of emphysema?
|
chronically irritated neutrophils and macrophages
|
|
|
changes seen in a PFT with emphysema? (3)
|
1. decreased FEV1
2. decreased FVC 3. increased RV |
|
|
a persistent cough with sputum production for at least 3 months in two years is known as?
|
chronic bronchitis
|
|
|
what would the Reid index be in a patient with chronic bronchitis?
|
increased
(more mucous and serous glands) |
|
|
in severe cases of chronic bronchitis there may be obliteration of the bronchial lumen, also known as?
|
bronchiolitis obliterans
|
|
|
In COPD, when is a person called a "blue bloater?"
|
when chronic bronchitis symptoms dominate
(also cor pulmonale, obese) |
|
|
in COPD, when is a person called a "pink puffer?"
|
when empysema symptoms dominate the disease
(thin, pursed lips) |
|
|
what is bronchiectasis?
|
chronic necrotizing infection of the bronchi and bronchioles leading to abnormal permanent dilatation.
|
|
|
what side does bronchiectasis most commonly occur on?
|
Left side
|
|
|
avg. age of onset of bronchiectasis?
|
first two decades
|
|
|
in what respiratory disease is bronchiectasis commonly seen?
|
CF
|
|
|
what is asthma that is refractory to aggressive treatment called?
|
status asthmaticus
(results in death) |
|
|
which antibody mediates Type I hypersentivity asthmatic reactions?
|
IgE
|
|
|
describe the acute phase response in a type I hypersensitivity asthmatic rxn
|
antigen exposure, binds to IgE receptor of mast cells
- results in release of mediators (histamine, leukotrienes, prostaglandins, cytokines)from the mast cells - these mediators trigger bronchiospasm, bronchoconstriction, edema, mucous secretion |
|
|
describe the late phase response.
|
seen 4-8 hrs. later
- caused by cells recruited in the acute phase (neutrophils, basophils, lymphocytes, eosin.) - additional mediator release and epithelial cell damage |
|
|
which drug is known to trigger asthma (along with triggering nasal polyps)
|
aspirin
|
|
|
what happens in exercise induced asthma?
|
physical activity induces bronchospasm
|
|
|
what causes microvascular pulmonary edema
|
usually injury related
- leakage of capillaries within alveoli |
|
|
histologically, what is seen in pulmonary edema as a result of heart failure?
|
hemosiderin laden macrophages in alveoli
|
|
|
describe a "saddle" pulmonary embolus
|
large thrombus at the bifurcation of the pulmonary artery - cuts off ALL pulmonary circulation. Cause of sudden death.
|
|
|
describe the symptoms seen in a peripheral embolus
|
sudden onset SOB, chest pain, tachycardia
|
|
|
what is the value that, above it, is called pulmonary hypertension?
|
25mm Hg
|
|
|
describe the morphology of pulmonary hypertension at the pulmonary artery level.
|
- hypertrophy of media of pumlonary arteries
- followed by intimal proliferation and fibrosis - plexiform lesions form in wall - these lesions dilate - results in hemorrhage and fibrinoid necrosis of the arteries and arterioles |
|
|
what is "pre capillary" pulmonary hypertension?
|
increased resistance in pulmonary arteries prior to capillary beds
|
|
|
causes of precapillary pulmonary hypertension? (3)
|
1. primary pulmonary HTN
2. L to R shunts 3. recurrent pulmonary emboli |
|
|
what is "post capillary" pulmonary hypertension?
|
increased resistance in post capillary vasculature
|
|
|
causes of post capillary HTN? (3)
|
1. LV cardiac failure (back up in lungs)
2. mitral stenosis 3. pulmonary veno-occlusive disease |
|
|
what type of antibodies are seen in goodpasture's syndrome?
|
anti-glomerular basement membrane antibodies
|
|
|
pulmonary manifestations of goodpastures?
|
hemorrhagic necrotizing interstitial pneumonia
|
|
|
what is the triad of symptoms seen in Wegeners Granulomatosis?
|
1. necrotozing angiitis
2. aseptic necrosis/granulomas of lungs and upper respiratory tract 3. glomerulonephritis |
|
|
Goodpastures and Wegeners are collectively known as?
|
diffuse Pulmonary Hemorrhagic syndromes
|
|
|
in relation to restrictive lung diseases, describe the:
1. oxygen diffusing capacity 2. lung volume 3. compliance 4. elasticity |
1. reduced oxygen diffusing capacity
2. reduced lung volume 3. reduced compliance 4. increased elasticity |
|
|
what is another name for most restrictive lung diseases?
|
interstitial lung disease (due to fibrosis)
|
|
|
the following disease belong to which class of lung diseases?
usual interstitial pneumonia (UIP) Desquamative intstitial pneumonitis (DIP) Bronchiolitis obliterans hypersensitivity pneumonia |
Diffuse Interstitial Lung Disease
|
|
|
of the diffuse interstitial lung diseases, which is the most common type?
|
UIP
|
|
|
of the diffuse intersitial lung diseases, which has the worst prognosis?
|
UIP
(87% mortality, avg. survival 103 yrs) |
|
|
what is the average age of onset of UIP?
|
5th-6th decade
|
|
|
UIP is also associated with an increased occurance of?
|
bronchogenic carcinoma
|
|
|
which area of lungs is most often involved in UIP?
|
base and pleural surface
|
|
|
In UIP, what is seen histologically?
|
heterogenous cellularity and fibrosis
|
|
|
grossly, what is seen in UIP?
|
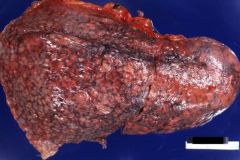
honeycomb change
knobby pleural surfaces ("muscular cirrhosis") |
|
|
what do we mean when we say temporal heterogeneity is seen in UIP?
|
cellular areas alternate with fibrotic areas
|
|
|
in desquamative interstitial pneumonitis (DIP), what is seen in the alveoli?
|
large aggregation of monocytes in alveoli
(also minor interstitial fibrosis) |
|
|
what would be seen in an X-ray of a DIP patient?
|
ground glass infiltrates
|
|
|
treatment for DIP?
|
steroids
(good response) |
|
|
which cell type is hyperplastic in DIP?
|
type II pneumocytes
|
|
|
what is bronchiolitis obliterans?
|
fibrosing inflammatory process that occludes the lumen of the small airways
|
|
|
what is BOOP?
|
bronchiolitis obliterans with organizing pneumonia
|
|
|
what is bronchiolitis obliterans?
|
fibrosing inflammation that occludes the lumen of the small airways
|
|
|
what are some possible etiologies of BOOP?
|
1. infections
2. toxins 3. obstruction 4. aspiration 5. collagen vascular disease |
|
|
which virus is often seen as a cause of BOOP?
|
RSV
|
|
|
describe the condition of the distal airways as seen in bronchiolitis obliterans
|
distal airways are overinflated and/or filled with cellular debris (macrophages, lipids, cholesterol)
|
|
|
what are some toxins that are known to cause bronchiolitis obliterans? (3)
|
1. nitrogen oxides (silofillers disease)
2. ammonia 3. acids |
|
|
T/F: a tumor can cause bronchiolitis obliterans
|
TRUE
tumors are considered obstructions that can cause bronchiolitis obliterans |
|
|
what causes hypersensitivity pneumonia?
|
prolonged exposure to dust allergens
|
|
|
how is hypersensitivity pneumonia different from asthma?
|
the reactive site is the alveolus
(the reactive site in asthma is the airway) |
|
|
what type of hypersensitivity rxn. occurs in hypersensitivity pneumonia?
|
early: Type III immune-complex rxn
late: type IV hypersensivity response |
|
|
describe the histopath seen in hypersensitivity pneumonia
|
granulomas frequently seen
macrophages, lymphocytes if chronic: fibrosis and honeycombing |
|
|
what are some allergens that oftentimes lead to hypersensitivity pneumonia?
|
bird dander
mold actinomycosis trichosporon much more... |
|
|
how is hypersensitivity pneumonia distinguished from pneumoconiosis?
|
hypersens. pneum: allergic reaction varies by individual
pneumoconiosis: all individuals react the same |
|
|
treatment for hypersensitivity pneumonia?
|
steroids
removal of allergen if possible |
|
|
what causes pneumoconiosis?
|
dust inhalation
|
|
|
what are some factors regarding the inhaled dust that lead to pathogenicity?
|
1. Amount of dust inhaled
2. Size and shape of dust particles 3. solubility and physiochemical properties 4. presence of additional irritants |
|
|
what is the most dangerous sized particle?
|
1-5 microns
these are the ones that reach small airways and alveoli |
|
|
what happens once the dust is inhaled that causes damage?
|
they produce a fibrotic response
|
|
|
which intrinsic defense mechanism removes inhaled larger particles?
|
mucociliary epithelium
|
|
|
which intrinsic defense mechanism is geared towards the particles that reach the alveoli?
|
alveolar macrophages
|
|
|
what is Caplans syndrome?
|
a triad of:
1. pneumoconiosis 2. rheumatoid arthritis 3. pulmonary rheumatoid nodules |
|
|
T/F: coal workers pneumoconiosis is associated with an increased cancer risk
|
FALSE
no increased CA risk |
|
|
describe the following stages of coal workers pneumoconiosis:
1. macule 2. nodule 3. complicated 4. progressive massive fibrosis |
1. aggregates of fibrosis with surrounding emphysema
2. fibrotic (>2.5 cm) 3. more than one lesion; symptomatic 4. end stage; large consolidated area; significant symptoms; RARELY OCCURS WITHOUT CONCURRENT Si OR TOBACCO EXPOSURE |
|
|
what are the terminal complications of pulmonary massive fibrosis? (2)
|
1. cor pulmonale
2. RV hypertrophy |
|
|
what is the most toxic form of inhaled silica?
|
crystalline silica (quartz)
|
|
|
describe acute silicosis
|
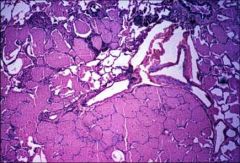
-rapidly develops, high mortality
-short term, high doses of small particle silica -diffuse fibrosis of lung; dense eosinophilic material in alveoli |
|
|
describe simple nodule (chronic) silicosis
|
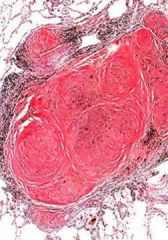
-pulmonary fibrosis WITH collagenous nodules
- concentric collagen bundles surrounding clear needle shaped spaces of silica - larger particles; longer, lower exposure |
|
|
chronic silicosis is associated with an increased risk of?
|
TB
(at least 30x) |
|
|
asbestos causes an increased risk of?
|
mesotheliomas
|
|
|
describe the morphology of asbestosis
|
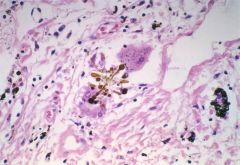
- diffuse interstitial fibrosis w/asbestos fibers
- circumscribed, dense, hyalinized or calcified plaques on pleura and diaphragm. |
|
|
what is siderosis?
|
iron dust exposure
(usually not significant) |
|
|
what is the difference between the acute and chronic forms of berylliosis?
|
1. acute - similar to an allergic response, no granulomas
2. chronic - non-caseating granulomas, diffuse interstitial reaction |
|
|
which two types of "bodies" are seen in berylliosis?
|
1. Schaumann body (protein, calcium, iron)
2. Asteroid bodies (lipoprotein aggregates in a giant cell) |
|
|
what is Loeffers Syndrome?
|
transient pulmonary lesions and eosinophilia; probably an allergic reaction
|
|
|
what is microfilaria?
|
when a worm or mite produces an eosinophilic response
|
|
|
describe the lung involvement seen in Scleroderma
|
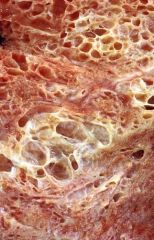
- diffuse fibrosis and vascular thickening
- lung involvement usually the fatal component of the disease - honeycombed changes |
|
|
result of pulmonary vascular intimal thickening seen in scleroderma?
|
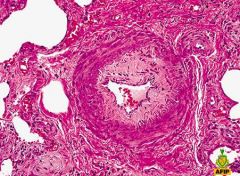
pulmonary hypertension
|
|
|
describe the lung involvement seen in RA
|
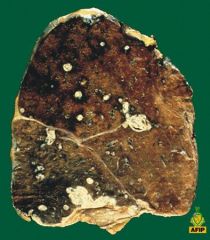
- rheumatoid nodules in lungs (especially pleural and subpleural locations)
- caplan's syndrome |
|
|
describe the lung involvement seen in Sjogren's syndrome
|
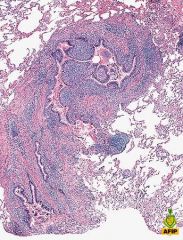
- lymphoid infiltrate in peribronchiolar and bronchial glands
- increase in lymphomas |
|
|
describe the lung involvement seen in SLE?
|
- infections and pleural effusions
- hemorrhage and vasculitis |
|
|
describe pulmonary alveolar proteinosis
|
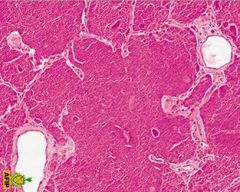
- protein deposits in alveoli
- few inflammatory cells - protein is similar to surfactant but without surfactant properties - thought to be an imbalance of phospholipid production and clearance |
|
|
describe the disease course of pulmonary alveolar proteinosis
|
1/3 resolve spontaneously
1/3 improve with pulmonary lavage 1/3 don't respond to any therapy |
|
|
stereotypical sarcoid patient?
|
adult 20-40 yrs
woman black |
|
|
sarcoid is characterized by?
|
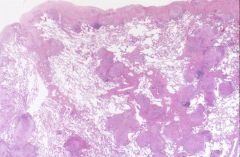
- non-caseating granulomas
- bilateral lymphadenopathy with hilar involvement |
|
|
besides the lungs, what other organs does sarcoid commonly effect?
|
heart
CNS skin oral cavity |
|
|
what is Lofgren's syndrome?
|
erythema nodosum
|
|
|
what is Mikulicz' syndrome?
|
bilateral sarcoidosis of lacrimal, sublingual, submaxillary, parotid glands
|
|
|
common histopath seen in sarcoidosis?
|
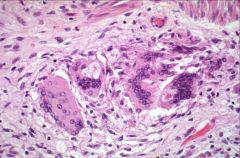
- giant cells containing asteroid bodies
- Schaumann bodies - GRANULOMAS |
|
|
which type of pulmonary carcinoma makes up >90%?
|
bronchogenic carcinoma
|
|
|
what age average does bronchogenic carcinoma most commonly occur in?
|
peak incidence in 6th and 7th decade
(rare before 40 yrs) |
|
|
80% of lung cancers occur in ?
|
smokers
|
|
|
give an example of radiation that increased rates of lung cancers.
|
Uranium miners
Atomic bomb survivors |
|
|
1. 20% of deaths in asbestos workers is due to?
2. 10% of deaths are due to? |
1. bronchogenic carcinomas
2. mesotheliomas |
|
|
what gas, found in the ground, is known to induce lung cancer?
|
Radon
|
|
|
which disease, associated with pulmonary scarring, has increased rates of lung cancer?
|
scleroderma
|
|
|
which type of lung carcinoma is the most lethal?
|
small cell carcinoma
|
|
|
what is the important distinction that must be made when trying to figure out the type of lung cancer? why?
|
small cell cs. non small cell
treatment differs: small cell considered nonresectable, others can be resected |
|
|
where do most bronchogenic carcinomas arise?
|
central airways
|
|
|
where do adenocarcinomas arise?
|
terminal bronchioles (Clara cells)
|
|
|
where do bronchioloalveolar carcinomas arise?
|
terminal bronchioles (Clara cells)
|
|
|
what is an:
1. exophytic lesion 2. endophytic lesion |
1. grows into lumen
2. grows into parenchyma |
|
|
where does lung cancer like to metastasize to? (4)
|
in order of preference:
1. adrenals 2. liver 3. brain 4. bone |
|
|
which type of lung cancer has a close correlation with smoking?
|
squamous cell carcinoma
(also small cell carcinoma) |
|
|
what histologic features are seen in squamous cell carcinoma? (2)
|
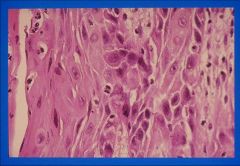
keratin
intercellular bridges |
|
|
where in the lung does squamous cell carcinoma usually occur?
|
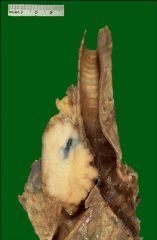
centrally (near hilum)
|
|
|
why do we see cavitating squamous cell carcinomas?
|
center of the tumor undergoes necrosis and cavitates
|
|
|
what is the most common cancer found in women and nonsmokers?
|
adenocarcinoma
|
|
|
where in the lung does adenocarcinoma tend to occur?
|

peripherally
|
|
|
what are the two histologic forms of adenocarcinoma?
|
1. typical bronchial derived adenocarcinoma
2. bronchioalveolar carcinoma |
|
|
which has a faster growth rate: adenocarcinoma or squamous cell carcinoma?
|
squamous cell carcinoma
|
|
|
where do bronchioalveolar carcinomas like to grow?
|
along pre-existing structures (such as alveolar walls)
|
|
|
what would bronchioalveolar carcinoma look like on Xray?
|
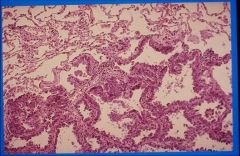
would look like infiltrates suggesting a pulmonary infection
|
|
|
treatment for bronchioalveolar carcinoma?
|
resection
(these are usually well-circumscribed tumors) |
|
|
small cell carcinoma is thought to arise from which cell type?
|
Kulchitsky's cells (neuroendocrine cells within the bronchial epithelium)
|
|
|
significance of small cell carcinoma arising from neuroendocrine cells?
|
tumor often secretes neuropeptides causing paraneoplastic syndromes
|
|
|
in SCC: tumor state at time of diagnosis?
|
generally have metastasized widely
|
|
|
what effects can some lung tumors have on the superior vena cava?
|
cause SVC syndrome (crush the SVC)
|
|
|
when the tumor causes a nerve entrapment syndrome: what symptoms present when the following nerves are trapped?
1. recurrent laryngeal n. 2. phrenic n. 3. sympathetic system |
1. hoarseness
2. diaphragmatic paralysis 3. Horners syndrome |
|
|
what is tylosis?
|
hyperkeratosis (callous formation)
caused by a type of paraneoplastic syndrome |
|
|
in metastatic lung cancer: when would you see leukoerythroblastosis?
|
when there is bone marrow involvement
|
|
|
what is the 5 yr survival rate of lung cancer in general?
|
<9%
dismal prognosis |
|
|
what would a bronchial carcinoid look like?
|
small projections into the lumen of the bronchus covered by bronchial epithelium
(good prognosis-metastisizes locally) |
|
|
what is responsible for most of the symptoms seen in bronchial carcinoid syndrome?
|
obstruction or hemoptysis
|
|
|
where are mucoepidermoid carcinomas most often found?
|
proximal bronchi or trachea
|
|
|
what is a hamartoma?
|
an abnormal overgrowth of tissues in an abnormal pattern (not malignant)
|
|
|
how can a malignant mesothelioma be distinguished from an adenocarcinoma?
|
by molecular markers
|
|
|
which virus am I?
- member of the herpesvirus family - causes pneumonitis that can result from a primary infection or from latent reactivation - has a 35-50% mortality rate in a bone marrow allograft patient that catches it? |
CMV
causes cytomegalovirus pneumonitis |
|
|
the hantavirus belongs to which virus family?
|
Bunyaviridae
|
|
|
describe the genome of the hantavirus
|
(-)ssRNA
trisegmented (3 separate RNA pieces) |
|
|
how is hantavirus transmitted?
|
aerosolized rodent urine
|
|
|
what are the three characteristics seen in Hantavirus Pulmonary Syndrome (HPS)?
|
1. pulmonary edema
2. shock 3. diuresis |
|
|
~ fatality rate of a hantavirus infection?
|
30-40%
|
|
|
which rodent is the primary carrier of sin nombre virus?
|
deer mouse
|
|
|
what would you see in the following lab values in HPS?
1. platelet count 2. lymphocytes 3. WBC differential 4. hematocrit |
1. low
2. atypical 3. Left shift 4. elevated |
|
|
what about these values in HPS?
1. albumin 2. LDH, AST, ALT |
1. low
2. elevated |
|
|
how is HPS managed?
|
EARLY:
1. aggressive supportive care (ventilation too) 2. inotropes (dobutamine) 3. careful monitoring of oxygenation, fluid balance, BP |
|
|
which test is used to diagnose influenza?
rationale? |
hemaglutination inhibition titer
*can see antibodies made in response to the flu |
|
|
which family of viruses does the influenza belong to?
|
orthomyxoviruses
|
|
|
which influenza strain causes pandemic flu and also infects other animals?
|
infuenza A
|
|
|
which influenza strain causes epidemic flu and only infects humans?
|
infuenza B
|
|
|
which influenza strain only causes mild disease and only infects humans?
|
influenza C
|
|
|
what two animals are important reservoirs for influenza A?
|
pigs
waterfowl |
|
|
describe the virion and nucleocapsids of influenza
|
enveloped virion
helical nucleocapsids |
|
|
describe the genome of influenza
|
segmented
(-)ssRNA |
|
|
1. influenza A and B have how many RNA segments?
2. what about influenza C? |
1. 8
2. 7 each segment encodes 1 or 2 gene products |
|
|
what are the two important antigenic determinants found on the influenza A surface?
|
Hemagglutinin (HA)
Neuraminidase (NA) |
|
|
function of hemagluttinin?
|
facilitates attachment to and fusion with host membrane
|
|
|
function of neuraminidase?
|
- allows penetration through respiratory secretions
- prevents viral aggregation |
|
|
what binds to viral RNAs and serves as a group specific antigen (A,B,C classification)?
|
neucleocapsid protein (NP)
|
|
|
what is the function of membrane protein (M1)?
|
matrix protein
binds to nucleocapsids during maturation |
|
|
what is the M2 protein?
|
an ion channel - important for uncoating
*activity inhibited by amantidine and rimantidine |
|
|
which receptors on the host cell does influenza A bind to?
|
scialic acid receptors
|
|
|
what causes HA to change its conformation?
what change occurs? |
acidification of the endosome causes conformational change
- change exposes hydrophobic residues for fusion |
|
|
acidification of the endosome also opens ____, which promotes uncoating.
|
M2 ion channel
|
|
|
what happens to the viral genome once uncoating occurs?
|
it is transported to the nucleus
|
|
|
because the RNA of influenza virus is (-), what is needed for viral gene expression?
|
cellular RNA polymerase
|
|
|
describe the production of mRNA in influenza A
|
1. viral polymerase with endonuclease activity is complexed to 5' end of host mRNA.
2. this 5' end is used as a primer for (-) viral RNA synthesis 3. this results in destruction of the host proteins: the cell will hereafter make viral proteins |
|
|
what are the 3 proteins the influenza viral polymerase is composed of?
|
1. PB1 (binds to cap of host mRNA)
2. PB2 (endonuclease) 3. PA |
|
|
does synthesis of the (+)RNA (antigenome)of influenza require priming by cellular mRNA?
|
NO
(only replication of the (-)RNA strand requires the primer) |
|
|
where are HA and NA inserted?
|
into the plasma membrane
(this is the site of virion budding) |
|
|
when do influenza epidemics occur?
|
winter or early spring
|
|
|
how often to influenza pandemics occur?
|
3-4 times a century
|
|
|
how is influenza transmissed?
|
respiratory droplets
|
|
|
is influenza a "hardy" virus?
|
YES
it remains infective for up to 24 hrs after aerosolization! |
|
|
what causes antigenic drift in influenza?
|
minor changes in the antigenicity of HA and NA (due to point mutations)
|
|
|
what causes antigenic shift in influenza?
|
reassortment of HA or NA RNA segments when two viruses infect the same cell (an entirely new gene is encoded)
|
|
|
significance of antigenic shift?
|
unpredictable
can lead to epidemics and/or pandemics |
|
|
why is antigenic drift so prone to occur in influenza?
|
because of an error-prone viral RNA-dependant RNA polymerase
|
|
|
there are 5 antigenic sites in the globular head of the H protein. how many antigenic sites must have mutations before antigenic drift occurs?
|
two or more sites must be mutated
|
|
|
why are pigs such a "scary" infuenza reservoir?
|
reassortment can occur in pigs: then the "new" virus subtype is transmitted to humans
|
|
|
mortality rate of H5N1?
|
51%
|
|
|
what is the incubation period of influenza virus?
|
1-5 days
(avg of 2) |
|
|
what effect does influenza have on mucous and how does this occur?
|
NA activity reduces viscosity of mucous allowing the virus to attach to cells
|
|
|
once the virus attaches to the cells, what happens?
|
desquamation of ciliary epithelium occurs (via degeneration of respiratory epithelial cells)
|
|
|
significance of impaired mucociliary escalator?
|
prevents extrusion of virus
increases susceptibility to secondary infections |
|
|
what leads to the cough and tracheal irritation?
|
tracheobronchial inflammation
|
|
|
if systemic spread is uncommon in an influenza infection: why is there myalgia and other systemic effects?
|
circulating cytokines cause these
(myalgia = IL-1 activated prostaglandin E2, which activates lysosomal proteases, which "chew" on muscle) |
|
|
how long after onset does it take for the fever and systemic effects subside?
|
3-5 days
|
|
|
what about the duration of respiratory complaints in an influenza infection?
|
cough etc. may persist for 2-4 weeks
|
|
|
complications of influenza? (3)
|
1. viral pneumonia
2. bacterial pneumonia 3. CNS manifestations |
|
|
what is the flu vaccine composed of?
|
2 A types
1 B types |
|
|
flu vaccine is recommended for?
|
ALL high risk groups >6 months of age
high risk includes: 1. adults >65 yrs 2. children 6-23 months 3. nursing home residents 4. individuals with pulmonary/CV disorders 5. children receiving long term aspirin therapy (prevent Reye syndrome) |
|
|
treatment for flu infection?
|
1. NSAIDs
2. amantidine, rimantidine (if <48 hours after onset, only for Influenza A) 3. neuraminidase inhibitors (Zamivir or oseltamivir) to reduce duration of symptoms |
|
|
which group of viruses do the following belong to?
RSV, parainfluenza, humanmetapneumovirus (hMPV), Mumps, measles |
paramyxoviruses
|
|
|
which paramyxovirus is a major cause of acute bronchiolitis and pneumonia in infants?
|
RSV
|
|
|
which virus is a major cause of croup?
|
parainfluenza
|
|
|
which virus is a common cause of respiratory disease in young children?
|
human metapneumovirus (hMPV)
|
|
|
which virus causes parotitis, orchitis, meningitis?
|
mumps
|
|
|
describe the virion and nucleocapsid structure of the paramyxovirus
|
enveloped virion
helical nucleocapsid |
|
|
describe the genome of the paramyxovirus
|
monopartite
(-)ssRNA |
|
|
describe the hemagglutinin and neuraminidase activities of the paramyxovirus
|
located on a single protein (HN)
|
|
|
do all paramyxoviruses have an HN protein?
|
NO
RSV lacks HN, but has G protein instead |
|
|
what is the function of the F protein in paramyxoviruses?
|
promotes virion-cell and cell-cell fusion
|
|
|
regarding paramyxovirus replication:
1. attachment to cell is via? 2. penetration is via? |
1. HN or G protein
2. F protein (endocytosis not required) |
|
|
regarding paramyxovirus replication:
1. what transcribes the genome to produce both mRNAs and the (+)ssRNA antigenome? 2. where does the entire replication cycle occur? |
1. virion RNA polymerase
2. cytoplasm |
|
|
in paramyxovirus: viral glycoproteins are expressed on the infected cell surface. what do these promote?
|
cell-cell fusion
production of giant cells (synctia) |
|
|
how do paramyxoviruses exit host cell?
|
budding from plasma membrane
|
|
|
what is the advantage of syncytia formation?
|
the virus can move from cell to cell directly and antibodies can't get at them while they are inside the cell
|
|
|
what general type of replication is seen in the paramyxoviruses?
|
start/stop replication
- gradient of expression of different proteins |
|
|
what is the most virulent type of parainfluenza virus (PIV)?
|
PIV-3
(causes croup, bronchitis, pneumonia in children <1yr) |
|
|
which type of PIV is usually asymptomatic?
|
PIV-4
|
|
|
describe the immunity gained after a PIV infection
|
short duration
re-infections are common but usually less severe |
|
|
1. when do PIV-1 and PIV-2 cause croup epidemics?
2. when does PIV-3 cause croup epidemics? |
1. Autum
2. late winter or spring |
|
|
transmission of PIV?
|
respiratory droplets
direct contact with fomites |
|
|
which type of PIV is known for nosocomial infections?
|
PIV-3
(most virulent type) |
|
|
1. incubation period of PIV
2. duration of shedding? |
1. 2-6 days
2. ~1 wk |
|
|
where does PIV normally multiply?
|
epithelial cells of URT
|
|
|
croup is characterized by?
|
barking cough
usually worse at night inspiratory stridor (with inspiratory retractions and tachypnea) respiratory distress |
|
|
three treatments used for symptomatic control in croup?
|
huimidification of inspired air
racemic epinephrine (reduces edema) dexamethasone (reduces edema) |
|
|
most important respiratory pathogen among infants and young children in temperate zones worldwide?
|
RSV
|
|
|
transmission of RSV?
|
direct contact
respiratory droplets fomites |
|
|
RSV causes the most severe infections in?
|
infants <6 months
|
|
|
when do annual epidemics of RSV occur?
|
midwinter
|
|
|
where does RSV replicate?
|
ciliated epithelial cells
(buds from apical portion of cell into lumen) |
|
|
RSV often causes plugs that obstruct airflow. where are these plugs found and what are they made of?
|
found in small airways
made of mucous, cellular debris, fibrin |
|
|
s/s of obstruction?
|
expiratory wheezing
focal atelectasis |
|
|
three severities of RSV infection?
|
1. mild URT disease
2. severe bronchiolitis 3. bronchopneumonia |
|
|
incubation period of RSV?
|
4-5 days
|
|
|
RSV can increase morbidity and mortality in adults with?
|
underlying conditions
COPD CHF asthma |
|
|
is RSV hardy?
|
NO
it is extremely labile - inoculate directly into cell culture when gathering a specimen |
|
|
there is a strong association between RSV bronchiolitis and ?
|
subsequent development of asthma
|
|
|
what can we do to decrease the number of RSV hospitalizatons in immunocompromised people?
|
give RSV antibodies once a month during RSV season
|
|
|
what is the name of a monoclonal anti-F antibody given to prevent RSV?
|
palivizumab
|
|
|
which virus presents and has a clinical course similar to RSV?
hint: it was discovered in 2001 |
human metapneumoviruses
|
|
|
what time of year is mumps epidemic in:
1. urban areas 2. rural areas |
1. mumps is endemic year round in urban areas
2. late winter and spring in rural areas |
|
|
how long does immunity to mumps last?
|
lifelong
|
|
|
how is mumps transmitted?
|
salivary secretions
respiratory aerosols |
|
|
where is the replication site for mumps?
|
URT epithelium
(from there it establishes viremia) |
|
|
incubation period of mumps?
|
18-21 days
|
|
|
mumps symptoms usually resolve in?
|
1 wk
|
|
|
complications of mumps? (3)
|
orchitis
meningitis deafness |
|
|
what type of vaccine is the mumps vaccine?
|
live attenuated
(part of MMR) |
|
|
four most common viruses that infect the URT?
|
in order of occurrence:
1. rhinovirus 2. coronavirus 3. enterovirus 4. adenovirus |
|
|
rhinoviruses are members of which viral family?
|
picornavirus
|
|
|
describe the virion and capsid of the rhinovirus
|
naked virion
icosahedral capsid |
|
|
describe the genome of the rhinovirus
|
small
(+)ssRNA genome (pico=small) |
|
|
the rhinovirus genome encodes what four structural proteins?
|
VP1-VP4
|
|
|
function of VP1
|
antigen for neutralizing antibodies
|
|
|
regarding rhinoviruses:
1. optimal growth temp? 2. optimal growth pH? |
1. 33 deg.C (below core body temp)
2. 6.8-7.3 |
|
|
implications of rhinovirus being acid sensitive?
|
inactivated at pH 3-5 (stomach acid)
this limits the infection to the URT |
|
|
which host receptors does the rhinovirus bind to? (2)
|
1. ICAM-1 receptors
2. LDL receptors |
|
|
does the rhinovirus undergo acidification in the endosome?
|
NO
the RNA is delivered directly into the cell |
|
|
general mechanism of rinovirus replication?
|
polyprotein expression
|
|
|
which protein directs the synthesis of (-)ssRNA antigenome and (+)ssRNA genome?
|
VPg protein
|
|
|
what must happen to the newly synthesized protein in order for the virus to become infectious?
|
viral protease must cleave VP0, generating VP2 and VP4
|
|
|
what are the two rhinovirus seasons?
|
early fall
late spring |
|
|
transmission of rhinovirus?
|
hand contact
self inoculation |
|
|
what is the usual portal of entry for rhinovirus?
|
nose
|
|
|
incubation period for rhinovirus?
|
1 day
SHORT! |
|
|
describe the pathogenesis of a rhinovirus infection
|
1. rhinovirus enters nose
2. transported by mucociliary action to nasopharynx 3. multiplies in nasopharyx 4. causes inflammation and increased nasal secretions |
|
|
where does rhinovirus replicate?
|
epithelium of nasal mucosa and nasopharynx
|
|
|
rhinovirus causes release of what inflammatory mediators?
|
histamine
prostaglandins ILs |
|
|
describe the mucous discharge as the rhinovirus infection progresses.
|
becomes more mucopurulent (due to increased presence of neutrophils)
|
|
|
why is a fever good in a rhinovirus infection?
|
increased temp limits replication of rhinovirus
|
|
|
how do you tell the difference between a rhinovirus infx and strep throat?
|
strep:
less nasal involvement less frequency of cough more severe pharyngeal discomfort |
|
|
how do you tell the difference between a rhinovirus infx and the flu?
|
flu:
more frequent occurrence of fever more severe malaise, myalgia, cough |
|
|
how do you tell the difference between a rhinovirus infx and secondary acute bacterial sinusitis (SABS)?
|
SABS:
very severe (meningitis, brain abcess) less severe cases present like a flu that won't go away |
|
|
what happens when you give ASA, antihistamines and NSAIDs.
|
you get symptomatic relief but you get a longer cold duration.
|
|
|
what are two enteroviruses that cause s/s similar to rhinovirus?
|
coxsackie A/B
Echovirus |
|
|
SARS belongs to what kind of virus group?
|
coronaviruses
|
|
|
which two antigenic groups of coronavirus are associated with the common cold?
|
229E
OC43 |
|
|
describe the virion and nucleocapsid of a coronavirus
|
enveloped virion with protruding glycoprotein spikes
helical nucleocapsid |
|
|
describe the genome of the coronavirus
|
LARGE
(+) ssRNA |
|
|
in the coronavirus: which protein is thought to function in receptor binding, viral cell fusion and as an antigen for neutralizing antibodies?
|
S (spike) glycoprotein
|
|
|
regarding coronaviruses: how is viral entry accomplished
|
via Fusion between the viral envelope and the plasma membrane
|
|
|
describe replication of the coronavirus
|
1. (+)ssRNA translated to produce RNA-dependant RNA polymerase
2. (-)ssRNA antigenome synthesized - serves as a template for production of subgenomic mRNAs. 3. mRNAs form a "nested set" with a common 3' end 4. S and HE glycoproteins are inserted into ER membranes and processed in the Golgi |
|
|
how are the virions formed?
|
via budding at the RER and Golgi
|
|
|
seasons for coronavirus infections?
|
winter
spring |
|
|
how often do coronavirus epidemics occur?
|
every 2-3 yrs
|
|
|
transmission of coronavirus?
|
respiratory route
|
|
|
difference in s/s between coronavirus and rhinovirus infections?
|
clinically indistinguishable
|
|
|
transmission of SARS-CoV?
|
close person to person contact
respiratory droplets |
|
|
SARS is thought to have originated from which animal?
|
bats
|
|
|
how long after incubation of SARS does lower respiratory phase begin?
s/s? |
3-7 days
(dry, nonproductive cough, dyspnea) |
|
|
where does SARS replicate?
|
outside of the lungs
(causes increased liver enzymes) |
|
|
at the peak of a SARS infection, describe:
1. WBC 2. platelet count |
1. low
2. low |
|
|
in which virus are 50% of infections asymptomatic?
hint: known to cause respiratory illness, ocular disease, gastroenteritis... |
adenovirus
|
|
|
describe the virion and capsid of the adenovirus infection
|
naked virion
complex icosahedral capsid - fibers at pentons serve as viral attachment proteins and important antigens - hexon protein also an important antigenic determinant |
|
|
describe the genome of adenovirus
|
LARGE
dsDNA 40 human serotypes (each associated with a different disease) |
|
|
is the adenovirus "hardy"?
|
yes
it is resistant to ether, alcohol and formaldehyde |
|
|
does uncoating of the adenovirus involve an endosome?
|
YES
|
|
|
where is adenovirus DNA transcribed?
|
nucleus
trancribed in two phases |
|
|
describe the early proteins involved in adenovirus replication
|
gene regulators
E1A - transcriptional activator EiB - prevents cell mediated apoptosis via p53 |
|
|
the late proteins are?
|
structural (capsid) proteins
|
|
|
which enzyme carries out DNA replication?
|
viral DNA polymerase
|
|
|
when do seasonal adenovirus infections occur?
|
late winter to early summer
|
|
|
what age group experiences the most adenovirus infections?
|
6 months to 5 yrs
|
|
|
transmission of adenovirus?
|
fecal-oral
direct contact aerosols ocular shedding |
|
|
different serotypes of adenoviruses are associated with what infections? (4)
|
1. endemic respiratory infections
2. epidemic respiratory infection 3. ocular infections 4. gastroenteritis |
|
|
latent period of adenovirus?
|
years
|
|
|
replication of adenoviruses occurs where? (2)
|
1. respiratory tract
2. GI cells |
|
|
regarding adenovirus
describe acute respiratory disease variant |
seen in military recruits
influenza like illness live oral vaccine (no longer used by army) |
|
|
describe the pharyngoconjunctival fever variant of adenovirus
|
children and young adults
transmitted in water one or both eyes infected associated with summer camps |
|
|
describe the epidemic keratoconjunctivitis variant of adenovirus
|
transmitted by contaminated hands, opthalmic instruments, solutions
one or both eyes can be infected pain, lacrimation, photophobia self limited, duration 4-6 weeks, vision unaffected |

