![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
120 Cards in this Set
- Front
- Back
|
The First Law of Thermodynamics
|
"Energy cannot be created or destroyed."
It can only change from one form to another. |
|
|
Mechanical Energy
|
Referred to as "work"
KE = 1/2 mv^2 m = mass v = velocity |
|
|
Thermal Energy
|

Referred to as "heat"
|
|
|
MAEB Lab: The system that is being described
|
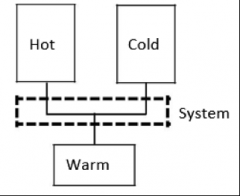
Where we have hot andcold water entering the system and warm water leaving the system.
|
|
|
The Mass Balance of the described system:
|
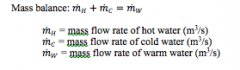
|
|
|
The Energy Balance of the described system:
|

|
|
|
DNA Lab: Step1 of the lab procedure says: “pipette 1 ml of the culture into amicrocentrifuge tube”. What is “the culture”?
|
“Theculture” is a liquid bacterium that was used to purify the plasmid.
|
|
|
DNA Lab: Step2 of the lab procedure centrifuges the sample. Is the DNA in the liquid orsolid?
|
TheDNA is in the solid.
|
|
|
DNA Lab: What does solution I do?
|
The Re-suspension solution containing Tris, EDTA, glucose,and RNase. Glucose maintains the osmotic pressure so the cells don’tburst and RNase A is included to degrade cellular RNA when the cells are lysed.
|
|
|
DNA Lab: Afteradding solution I and shaking or flicking, is the DNA in a liquid or a solid?
|
After adding solution shaking, and I the DNA is in a liquid.
|
|
|
DNA Lab: What does solution II do?
|
The alkaline lysis solution contains NaOH, which breaks down the cell wall, andSDS, which breaks down the cell membrane and abolishes the proteins in the cell. And DNA to strand.
|
|
|
DNA Lab: After adding solution II and gently mixing the solution, is the DNA in a liquid or solid?
|
Proteins and junk are solid DNA dissolved
|
|
|
DNA Lab: What does solution III do?
|
Solution III contains potassium, which neutralizes the pH.
|
|
|
DNA Lab: Why does the sample become cloudy?
|
The solution becomes cloudy due to the insoluble cell fragments in the liquid.
|
|
|
DNA Lab: Step 10 of the lab procedure centrifuges the sample for 10 minutes. Why is it important to avoid pipetting any solid to the spin column?
|
It is important to avoid pipetting any solid to the spin column because the solid contains only debris and waste that could taint the solution.
|
|
|
DNA Lab: How would you explain to a stranger what a “spin column” is?
|
A spin column contains a silicon membrane that binds theDNA.
|
|
|
DNA Lab: What is the purpose of the spin column in the procedure?
|
The purpose of the spin column in the procedure is to purify the DNA.
|
|
|
DNA Lab: Insteps 15-18, the column is “washed”. Where is the DNA during the wash?
|
The DNA is located in the spin column membrane.
|
|
|
DNA Lab: In step 22, an elution buffer is added to the solution. What does the elution buffer do?
|
The elution buffer removes the DNA from the filter, and the DNA is collected.
|
|
|
DNA Lab: After the sample is centrifuged in step 24, where is the DNA?
|
The DNA is now in its purified form.
|
|
|
DNA Lab: What was your DNA concentration?
|
The DNA has a concentration of 31.5 ng/uL liquid.
|
|
|
DNA Lab: What is a DNA ladder?
|
A DNA ladder is a set of DNA fragment values that are in separated bands in a gel matrix in the shape of a ladder.
|
|
|
DNA Lab: Why is a DNA ladder used in gel electrophoresis?
|
The DNA ladder is used in gel electrophoresis to monitor the DNA migration for linear DNA fragments as a function of their length.
|
|
|
DNA Lab: When working with live cultures, like E. Coli.,where should waste NEVER go?
|
When working with live cultures, like E. Coli,waste should NEVER go in the trash or a sink.
|
|
|
Glucoamylase (GA)
|
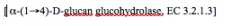
-An ubiquitous enzyme among all vertebrate,plants, and diverse microorganisms.
- It catalyzes the production of glucose from the non-reducing end of starch and maltooligosaccharides. |
|
|
What is an Enzyme?
|
A protein that can catalyze a specific reaction
|
|
|
EC 3.2.1.3
|
-Is called an Enzyme Commision Number
-Defines the function of the enzyme -1st Number (3): Type of Reaction -2nd Number (2): Substrate -3rd Number (1): Catalytic Action -4th Number (3): Specific details for the reaction |
|
|
Hydrolyzes
|
GA Interacts with Starch to make Glucose
|
|
|
What is Starch?
|
Long polymer chain of glucose molecules
|
|
|
Structure of Amylose (Starch)
|
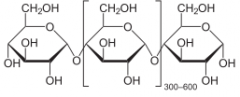
-Middle Section: Repeating Units
|
|
|
What does it mean to HYDROLYZE STARCH?
|
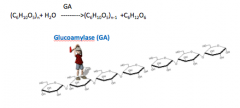
Cleave a glucose molecule from the end:
GA catalyzes the production of D-glucosefrom the non-reducing end of starch and maltooligosaccharides, cleaving both a-(1-4) or a-(1-6) glucosidic bonds. |
|
|
Why would we want to Hydrolyze starch?
|
Glucose is an optimal substrate for a fermentation process
-EXs: biofuels, high fructose corn syrup, 1,3 propanediol, etc |
|
|
Why not just feed a fermentation glucose?
|
Hydrolyzing starch is cheaper
|
|
|
Where does starch come from?
|
We harvest this mostly from plants (e.g. corn starch)
|
|
|
Why do plants make starch in the first place?
|
-Effective method of storing food reserves
-Microbes would degrade a glucose storage reserve -Most microbes cannot degrade starch natively (some can) |
|
|
The Enzyme Assay:
|
-We want to measure the amount of glucose present----> as time increases, glucose increases
-Principles of the assay (how to measure glucose?) |
|

Principle of Enzymatic Glucose Assay Method:
|
-Glucose is phosphorylated by adenosinetriphosphate (ATP) in the reaction catalyzed by hexokinase.
- Glucose-6-phosphate (G6P) is then oxidized to 6-phosphogluconatein the presence of oxidized nicotinamide adenine dinucleotide (NAD) in areaction catalyzed by glucose-6-phosphate dehydrogenase (G6PDH). - During this oxidation, an equimolar amount of NAD isreduced to NADH. The consequent increase in absorbance at 340 nm is directlyproportional to glucose concentration. *NADH is easily measured using absorbance at 340 nm |
|
|
Michaelis - Menten enzyme kinetics
|

|
|
|
Ifthe substrate concentration is far lower than Km, [S] can be omitted in thedenominator. The equation will reduceto:
|
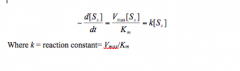
|
|
|
Solving a differential equation -d[S]/dt
|
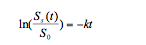
y=mx
-Plot y-axis: ln(S/S0) -Plot x-axis: t Plot will produce a straight line. Slope = -k intercept = 0 |
|
|
Calculating Delta A
|
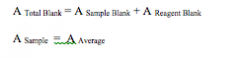
|
|

Calculating Glucose Concentration:
mg glucose/ml |

|
|
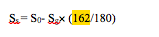
Starch Concentration
|

|
|
|
What is a Protein?
|
1.Polymers composed of amino acid monomers linkedtogether by peptide bonds forming a polypeptide chain.
2.They are linear polymers without branches 3.Each protein has a unique structure todifferentiate itself from all other proteins. 4.Proteins that are used as drugs are calledbiopharmaceuticals. 5.DNA codes proteins 6. DNA ->RNA -> Proteins |
|
|
What is a Polymer?
|
1.A large molecule (Macromolecules) composed ofmany similar smaller molecules (monomers) linked together.
2.Responsible for the molecular"uniqueness" of an organism |
|
|
What is an Amino Acid
|
Amino acids are a simple organic compoundcontaining both a carboxyl (—COOH) and an amino (—NH2) group.
|
|
|
10 Amino Acids that Human can produce:
|
1. Alanine(Small)
2.Asparagine (Amide) 3. AsparticAcid (Acidic) 4. Cysteine(Nucleophilic) 5. GlutamicAcid (Acidic) 6.Glutamine (Amide) 7. Glycine(Small) 8. Proline(Hydrophobic) 9. Serine(Nucleophilic) 10.Tyrosine (Aromatic) |
|
|
The ESSENTIAL AminoAcids Required in the diet:
|
1. Arginine(required for young, but not for adults) (Basic)
2.Histidine (Basic) 3.Isoleucine (Hydrophobic) 4. Leucine(Hydrophobic) 5. Lysine(Basic) 6.Methionine (Hydrophobic) 7.Phenylalanine (Aromatic) 8.Threonine (Nucleophilic) 9.Tryptophan (Aromatic) 10. Valine(Hydrophobic) |
|
|
Amino Acids' Role in Proteins
|
-Proteins not only catalyze all (or most) of thereactions in living cells, they control virtually all-cellular process.
-In addition, proteins contain within their aminoacid sequences the necessary information to determine how that protein willfold into a three dimensional structure, and the stability of the resultingstructure. |
|
|
Significance of Amino Acids
|
- Failure to obtain enough of even 1 of the 10essential amino acids, those that we cannot make, results in degradation of thebody's proteins—muscle and so forth.
-Unlike fat and starch, the human body does notstore excess amino acids for later use—the amino acids must be in the foodevery day. |
|
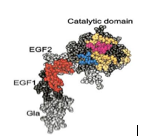
Protein Structure
|
-One or more polypeptide chains twisted into a3-D shape form a protein
-Chemical bonding between portions of thepolypeptide chain aid in holding the protein together and giving it its shape |
|
|
Protein Production
|
1.Proteins need to be expressed by a biologicalhost – Expression Host
2.The process to insert a gene of interest into anexpression host is called recombinantDNA technology 3.Protein expressed by a host, which does notnaturally produce the protein, is called RecombinantProtein |
|
|
Protein Expression
|
1. Many Expression hosts:
o Bacteria, such as E. coli o Yeast o Mammalian Cell 2. Other Expression hosts: o Transgenic Plants: corn, potatoes, tomatoes o Transgenic Animals: Sheep, goats, chicken eggs,cows |
|
|
Protein Purification
|
1. The mostcostly process for protein production
2. Typically many unitoperations are needed to purify a protein from a complex feedstock, which contains other proteins, DNAs, RNAs,and other molecules 3. The ensemble of the unitoperations is called a protein purification process |
|
|
Chromatography
|
The materials inside the column is calledresins, which could have different chemical or biochemical ligands attached tothem to enable specific interactions with certain molecules – stationary phase Proteins to be separated by chromatography arein a liquid solution – mobile phase |
|
|
Chromatogram
|
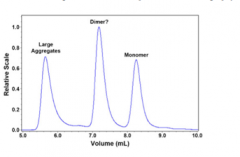
A chromatogram is the visual output of the chromatography operation
|
|
|
Detection of Protein
|
The presence of protein in a liquid solution isoften determined by measuring the absorbance at 280 nm because all proteins(and many other compounds) absorb ultraviolet (UV) light
|
|
|
Detection of Protein Visual
|
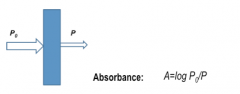
|
|
|
The Beer - Lambert Law
|

|
|
|
Protein Concentration
|
* Proteinmolecular weight
-Lysozyme’sMW = 14,400 Da (Dalton) * Alysozyme solution has a concentration of 1 mg/ml * Calculatethe concentration in mol/L |
|
|
Mass Balances in Chromatography
|
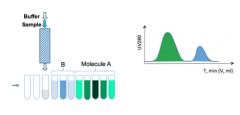
|
|
|
Mass Balances in Chromatography Cont'd
|

1. Determine the protein concentration in each test tube(fraction), which contains the target protein.
2. Calculate the total protein recovered: 3. Calculate the recovery (yield) of a proteinafter a separation operation: |
|
|
Gel Filtration Chromatography
|
-Method to separate desired proteins from mixture
-GelFiltration – separate molecules by size |
|
|
Protein Recovered
|

|
|
|
Why is water such a big deal?
|
Water is essential to life60-80% of the human body is waterMultiple needs: drinking, washing, agriculture, cleaning, industry, etc.
Water is a frighteningly effective disease vector “Contaminated water can contact many more people than an infected person can…the more waterborne the diarrheal bacterium, the more deadly it is.” ~Paul Ewald, Plague Time: The New Germ Theory of Disease |
|
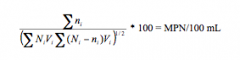
Thomas Equation
|
n = number ofpositive wells for each dilution
N = totalnumber of wells for each dilution V = volume ofa single well for each dilution |
|
|
Mass-Balance (Flow Rates)
|
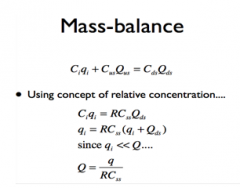
|
|
|
Flow Rate Approach
|

|
|
|
Flow Rate Relative Concentration
|

|
|
|
Flow Rate Determining K
|
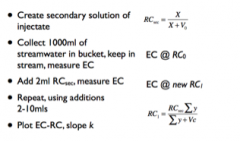
|
|
|
Flow Rate Sample Calculation
|

|
|
|
Discharge
|
The volume of water flowing through a cross section of a stream channel per unit time. *The most fundamental of hydrologic measurements that characterize all river and stream ecosystems. |
|
|
Velocity - Area Method
|
Involvesmeasuring water depth and velocityat points across a stream section witha current meter.
*The most common approach to measuring discharge. |
|
|
Physical Processes that Contribute to Breakthrough Curves
|
tracer mass recovered, mean residence time, mean flow velocities,longitudinal dispersion,depth, and storage
|
|
|
Breakthrough Curve from Stream Tracer Lab
|
The breakthrough curve displayed in Figure 2 resembled a bell-curved, showing a Gaussian distribution of the plateau concentration.
This curve demonstrates relative concentration running in the uniform course of flow. |
|
|
Stream Tracer Intro
|
1. In this laboratory, wewill use a conservative solute injection technique to quantify flow rate withina stream channel.
2. A conservative(nonreactive) tracer (e.g. NaCl) will be injected into a stream channel at aknown flow rate, qi. 3. Electricalconductivity (EC) is a proxy for the salt concentration. 4. By measuring EC priorand during the injection, we can determine the stream flowrate Q. |
|
|
Whatquestion(s) was the study authors attempting to answer with this researcheffort?
|
-Whatrelationships exist between the presence of enteric bacteria and demographicfactors, such as land use?
-Whateffect did different physical and chemical water quality factors have in thepresence of enteric bacteria? |
|
|
The authors state onp. 1048: “An advantage to the goals of our investigation was that the fiveseparate estuarine watersheds targeted for study were similar in climate,geography, and soil type because of inter-watershed proximity”. Why was itimportant to keep these factors relatively constant?
|
-It was needed to ensure that any varyingdata was a result of what was being tested. -Eliminating as many outside variables aspossible allows for procuring certainty so any discrepancy present in data is aresult of the testing inconstant.
|
|
|
What is “turbidity”? Table 3 on p. 1050indicates that it was strongly positively correlated with fecal coliform and E. coli concentrations. Why would thisbe so?
|
-Turbidity is a measure of cloudiness ofwater based on how many particles are suspended in the water.
-There would be a high correlationbetween bacteria counts and turbidity due to increase in turbidity holding agreat share in the aftermath of a storm water runoff from a surrounding human –inhabited area. |
|
|
Table 5 reveals a statisticallysignificant relationship between fecal coliform levels in streams and totalland area and human population. Where might these bacteria be coming from?
|
-There are a number of fecal coliformsources present in highly populated areas including wild and domestic animalfeces, agricultural runoff from fields, and waste from sewage plants.
|
|
|
5. On p. 1053 of the discussion theauthors assert: “the quality, rather than quantity, of land development is themost important influence on urban and suburban nonpoint source fecal coliformbacterial pollution.” What do the authors mean by “quality land development”?How would these affect bacteria concentrations in streams?
|
-This meant that quality land development creates moreimpervious surface where water was unable to enter into the ground and leavebehind fecal matter.
-The water would instead flowmore directly into the stream and rivers, bringing with it any fecal matter itflowed over on impervious surfaces like concrete. |
|
|
Fecal Indicator Bacteriaand Anthropogenic Influences LaboratoryGOAL:
|
The overall goal of this laboratory is touse observational fecal indicator bacteria data to identify five “mystery”water samples
|
|
|
Five Mystery Water Samples
|
·New River in Radford, VA
·Pandapas Pond in GilesCounty ·Seitz Hall bathroom sink ·Vet-Med Pond on VTCampus ·Duck Pond on VT Campus |
|
|
Colilert Defined Substrate Method
|
-Colilert* simultaneously detects total coliforms and E. coli in water.
-When total coliforms metabolize Colilert’s nutrient-indicator, ONPG, the sample turns yellow. -When E. coli metabolize Colilert’snutrient-indicator, MUG, the sample also fluoresces. -Colilert can simultaneously detect these bacteria at 1 cfu/100 mL within 24 hourseven with as many as 2 million heterotrophic bacteria per 100 mL present. |
|
|
Sample # 4
0,0 Coliforms & 0,0 E.coli |
The sink is likely to have the lowest concentration of fecal indicators, as the water was run through human sanitation systems and cleaned extensively.
|
|
|
Sample # 5
49,29 Coliforms & 7,2 E.coli |
-The duck pond, which was exclusively designed to attract ducks and other waterfowl, is likely to have one of the highest fecal indicator concentrations due to it attracting large populations of ducks.
-The duck pond was originally built as a storm water retention pond, fed by Stroubles creek. This means that all of the bacteria picked up by Stroubles creek is given to the duck pond and held there. |
|
|
Sample # 1
48,15 Coliforms & 19,2 E.coli |
-Pandapas pond is a manmade pond that is not an impaired body of water and is open for recreational use for the general public.
-Therefore it is not very likely to have large concentrations of fecal indicators. |
|
|
Sample # 2
38,9 Coliforms & 29,7 E.coli |
-According to a report released by the Virginia Department of Environmental Quality (VDEQ) sections of the New River and many runs and creeks in the New River Basin are considered impaired for E. Coli and high Mercury concentrations as of 2014.
-Since the New River runs along a very rural area, there are many farms and wild lands to allow for fecal matter to wash into its water and so it would likely have a high coliform concentration. |
|
|
Sample # 3
48,11 Coliforms & 43,2 E.coli |
Finally, the Vet-Med Pond would fall into a similar category to the duck pond, as it is a still body of water. |
|
|
Microbiology and Public Health
|
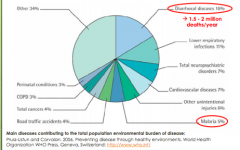
|
|
|
Whatare some examples of water contaminants of human health concern?
|
-Arsenic
-Fluoride -Selenium and Uranium -Cyanide -Lead -Inorganic Mercury |
|
|
Prokaryotic Cells
|
singlecelled organisms with no membrane-bound organelles (i.e. no ‘realnucleus’)(2-4 μm)
|
|
|
Eukaryotic Cells
|
cellswith a true membrane-bound nucleus (> 5 μm)
|
|
|
Viruses
|
Obligateintracellular parasites (20-300nm)
|
|
|
Where are microorganisms essential?
|
Withinour bodies
Withinnatural ecosystems Withinengineered systems |
|
|
Pathogen
|
An infectious microorganism; an etiological agent of chronic disease.
- Several distinct pathogens identified - New diseases are emerging |
|
|
How do pathogens get into our water?
|
Sanitation= provisionoffacilities and services for the safe disposal of humanurine and feces
|
|
|
TheFecal-Oral Exposure Route
|
-Is a route of transmission of a disease, when pathogens in fecal particles passing from one host are introduced into the oral cavity of another host.
-One main cause of fecal-oral disease transmission in developing countries is lack of adequate sanitation. |
|
|
Where are thepotential sources of fecal contamination?
|
Wastewater treatment plants
On-site septic systems Domestic and wild animal manure Storm runoff |
|
|
Howdo we monitor microbial contamination?
|
Members of two bacteria groups, coliforms and fecal streptococci, are used as indicators of possible sewage contamination because they are commonly found in human and animal feces.
|
|
|
Fecal Indicator Organisms
|
used in the detection andmonitoring of sewage; sentinels of
|
|
|
Characteristics of an Ideal indicator Organism
|
-Occurswhere pathogens do
-Canbe isolated from all types of water -Easyto isolate and count -Cannotgrow in the environment -Moreresistant to disinfection than are pathogens -Foundin higher numbers than pathogens -Nonon-fecal sources -Densityshould relate to human health risk |
|
|
Coliforms
|
Gram-negative,non-sporulating,rod-shaped bacteria that ferment lactose; native mammalian intestinal flora
|
|
|
NOTE****
|
ALL Virginia surface waters are classified as
“primarycontact” |
|
|
States of the Waters
|
Theleading cause of impaired surface waters in the United States is elevatedconcentrations of fecal indicator bacteria.
|
|
|
Surface Water Body Impairments
|
-Diseaseoutbreaks associated with drinking water have decreased
*HOWEVER,water demand is increasing * Treatmentcosts money and energy * Emergingchlorine-resistant pathogens -Diseaseoutbreaks associated with recreational water usage have increased -Severalrecent food-borne outbreaks associated with irrigation waters |
|
|
Process vs. Principle
|

|
|
|
BSE Mission Statement
|
The mission of the Biological Systems Engineering Department is to develop and disseminate engineering knowledge and practices that protect natural resources and improve sustainable production, processing, and utilization of biological materials.
|
|
|
BSE Design Principles
|
1.Identify,quantify, and manage risk, including the risk of inaction.
2. Clearlycommunicate sources of uncertainty and inherent design limitations (includingdesign life).
3.Exercise soundleadership, ethics, management, and stewardship in decision-making processes. 4. Employ anintegrated systems approach thatincludes social, environmental, and economic considerations. 5. Promote theuse of biological materials and processesto devise sustainable solutions andprotectnatural resources. |
|
|
1)Purpose of Identifying need
|
Understandingthe client’s demand
▪Need: customerwants something fun in the local park |
|
|
2) Purpose ofdefining the problem
|
Develop cleardefinition of the problem
▪Question: Whatis our problem? |
|
|
3) Purpose of Search
|
Learn what’sbeen done, mistakes made, possible improvements
▪Example:internet ▪Question:Where can we search? |
|
|
4) Purpose of Constraints
|
Identifylimitations (physical limits, cost, performance, etc.)
▪Example:design must be delivered by July 2015 ▪Question: Whatare some constraints in designing our item? |
|
|
5) Purpose of Criteria
|
Identifydesirable characteristics for the design
▪Example: theitem should be durable ▪Question: Whatare some criteria that we would want for our design?
|
|
|
6) Alternative Solutions
|
▪Purpose:generate multiple solutions
▪Example: slide ▪Question: Whatare some alternative solutions that meet our constraints and criteria? |
|
|
7) Analysis
|
▪Purpose ofanalysis: use mathematical, engineering, market principles, and common sense,to assess the solutions
▪Examples: putdesign under load ▪Swing design -What types ofanalysis can you perform? |
|
|
8) Decision
|
▪Purpose ofdecision: determine the optimal solution
▪Example –Usedecision matrix to choose the swing design that meets constraints and bestmeets criteria |
|
|
9) Specification
|
▪Purpose:provide details of design
▪Example:materials, industry standards required ▪Swing design:based on constraints and criteria, what specifications would you make in yourdesign? |
|
|
10) Communication
|
▪Purpose:convey the problem/need, decision-making process (constraints, criteria, etc.),final decision, and details of final design
▪Examples: oralpresentation, written reports |
|
|
DESIGN LAB
|
▪Objective:design a sedimentation tank that will settle kaolinite (a type of clay) quicklyand cheaply
▪In lab: smallscale sedimentation experiments ▪Design: largescale sedimentation facility ▪Dimensions oftank(s) ▪Come up withconstraints, criteria, and specifications for your sedimentation design *and*that consider the scenario provided |

