![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
402 Cards in this Set
- Front
- Back
|
the cytoplasmic granularity found in neurons is called?
|
Nissl substance
|
|
|
what type of stains are used for the best visualizations of neurons?
|
silver-based stains
("Beil" stain, Bodian stain) |
|
|
T/F: neurons have no regenerative capacity
|
True
|
|
|
under what conditions would a neuron undergo chromatolysis?
|
when the axon is severed
(chromatolysis = swelling of cell body) |
|
|
a neurological rabies infection would have what microscopic neuronal manifestation?
|
inclusion bodies
|
|
|
describe the microscopic characteristics of ischemic change in a neuron
|
pyknotic nucleus
red cytoplasm "red-dead neurons" |
|
|
what occurs in chromatolysis and what are the microscopic characteristics of it?
|
chromatolysis - severing of axon
see an eccentric nucleus, ground glass cytoplasm |
|
|
function of astrocytes?
|
transport substances from blood to CNS
|
|
|
useful stains that help us visualize astrocytes? (3)
|
PTAH
GFAP immunostain |
|
|
MC reaction of astrocytes to injury?
|
proliferation, cause gliosis (glial scar)
|
|
|
what is the "gemistocytic change" sometimes seen in neuronal injury?
|
astrocytes that are plump, round and have an abundant eosinophilic cytoplasm
|
|
|
when are Alzheimer's type II astrocytes seen and what do they look like?
|
seen in hepatic failure
swollen, bizarre nuclei |
|
|
when are Rosenthal fibers seen?
|
in chronic gliosis
|
|
|
stain used to best see myelin?
|
luxol-fast blue
|
|
|
reaction of oligodendrocytes to injury?
|
most likely cell death with resulting myelin loss
|
|
|
where are ependymal cells found?
|
line the ventricular cavities and the neural tube
|
|
|
function of choroid plexus?
|
produces CSF by ultrafiltration
|
|
|
changes seen in the ependymal lining when there is underlying astrocytic proliferation going on?
|
ependymal granulations
|
|
|
describe the ependymal cell
|
ciliated cuboidal epithelial cell
|
|
|
function of microglial cells?
|
specialized CNS macrophages
|
|
|
what is the stimulus for transformation of microglial cells to "rod cells?"
|
injury causes proliferation of microglial cells with elongation of nuclei = rod cells
|
|
|
what reaction does a microglial cell have to a virally infected neuron?
|
attacks it
|
|
|
how does a microglial cell become a foamy macrophage?
|
it scavenges lipid in infarcted and demyelinated areas
|
|
|
what stain is used to best visualize lipid-laden microglial cells?
|
oil-red-O stain
(orange is the lipid scavenged by the macrophages) |
|
|
what are the four horizontal levels of a neuropathological lesion?
|
1. supratentorial
2. posterior fossa 3. spinal 4. peripheral |
|
|
what are the 4 basic differentiations we should make in the clinic to evaluate a neurological problem?
|
1. anatomic level of lesion
2. focal or diffuse 3. progressive or nonprogressive 4. acute, subacute, chronic |
|
|
most acute neuropathological incidents are of what etiology?
|
vascular
|
|
|
give an example of a focal, acute neuropathological incident
|
infarct
intraparenchymal hemorrhage |
|
|
most subacute neuropathological incidents are of what etiology?
|
inflammatory
|
|
|
give an example of an acute, diffuse, neuropathological incident
|
subarachnoid hemorrhage
anoxia |
|
|
give an example of a focal, subacute neuropathological etiology
|
abscess
|
|
|
give an example of a diffuse, subacute neuropathological etiology
|
meningitis or encephalitis
|
|
|
what is the most likely etiology of chronic, focal neuropathological symptoms?
|
neoplasm
|
|
|
what is the most likely etiology of chronic, diffuse neuropathological symptoms?
|
degenerative
|
|
|
what are the proposed mechanisms behind demyelinating disease? (3)
|
1. inflammatory (immune, autoimmune)
2. genetic 3. unknown |
|
|
what type of demyelinating disease is characterized by a relapsing and remitting course?
|
MS
|
|
|
is MS characterized by focal or diffuse deficits?
|
focal
(distinct demyelinating lesions in the CNS) |
|
|
when is the typical onset of MS?
|
3rd-4th decade
|
|
|
F:M of MS?
|
F:M = 2:1
|
|
|
what pathways are impaired in MS?
|
visual
sensory motor coordination |
|
|
two types of MS?
|
1. classic (Charcot) form
- most common, classic relapsing and remitting course with gradual overall decline 2. acute (Marburg) form - young adults, rapidly progressive and unrelenting course |
|
|
MS is most apparent in what portion of the brain and spinal cord?
|
white matter
(in MS you see gray matter plaques in white matter areas) |
|
|
what is a shadow plaque?
|
an older MS plaque that shows limited remyelination
|
|
|
microscopic characteristics of the axons in MS?
|
axons are relatively spared so they are most likely "normal" looking
|
|
|
where in the brain is it common for MS plaques to appear?
|
around the ventricles (esp. the lateral horns of the lateral ventricles)
|
|
|
describe the borders of an MS plaque
|
well circumscribed borders
|
|
|
describe the immune pathogenesis of MS that Dr. Weiland gave.
|
* oligodendrocyte injury mediated by cytotoxic CD4+ T-cells
* see oligoclonal immunoglobulins in the CSF * lymphocytic infiltrates in the plaques |
|
|
is there a genetic link for MS?
which HLA is MS associated with? |
* increased risk in 1st degree relatives, increased in twins
* associated w/HLA-DR2 |
|
|
geographically, where is MS more common?
|
temperate zones
(risk conferred by 18 yrs of age) |
|
|
which demyelinating disease is characterized by prominent visual and spinal cord involvement and NO cerebral or cerebellar involvement?
|
neuromyelitis optica (Devic's disease)
|
|
|
how can acute disseminated encephalomyelitis (ADEM) be distinguished from MS?
|
ADEM is a diffuse demyelinating autoimmune disease, MS is a focal demyelinating autoimmune disease
|
|
|
common etiologies of ADEM?
|
* often occurs after childhood viral illness (measles, rubella, VZV)
* vaccinations (rabies, mumps, rubella) |
|
|
microscopic characteristics of ADEM?
|
perivascular inflammatory infiltrates with demyelination (seen in entire brain)
|
|
|
prognosis of ADEM?
|
variable, but most mild cases completely recover
|
|
|
describe acute hemorrhagic leukoencephalitis (AHL)
|
"hemorrhage with demyelination"
(a hyperacute form of ADEM with hemorrhage - often fatal within days) |
|
|
micro characteristics of acute hemorrhagic leukoencephalitis? (4)
|
* perivascular demyelination
* inflammatory infiltrates * fibrinoid necrosis * punctate hemorrhages |
|
|
symmetric non-inflammatory demyelination of the pons is known as?
|
central pontine myelinolysis
|
|
|
possible etiology of central pontine myelinolysis?
|
* hyperosmolality due to rapid overcorrection of hyponatremia
|
|
|
clinical features of central pontine myelinolysis?
|
ranges from asymptomatic to flaccid quadraplegia to stupor or coma.
|
|
|
leukodystrophies are hereditary disorders of?
|
myelin production or metabolism
|
|
|
metachromatic leukodystrophy is a defect in what enzyme? result?
|
defect in arylsulfatase A
*results in accumulation of sulfatides in oligodendrocytes |
|
|
Krabbe disease is a defiency in?
|
beta-galactosidase
|
|
|
Adrenoleukodystrophy results in?
|
high levels of very long chain fatty acids
|
|
|
inheritance pattern of leukodystrophies?
|
AR
|
|
|
inheritance pattern of neuronal storage diseases?
|
AR
|
|
|
Tay-Sachs is a deficiency in?
|
hexosaminidase A
*result is accumulation of gangliosides |
|
|
Hurler syndrome is a deficiency in?
|
a-iduronidase
* result is accumulation of mucopolysaccharides |
|
|
Gaucher's disease is a deficiency in?
|
glucocerebrosidase
|
|
|
Neimann-Pick syndrome is a deficiency in?
|
sphingomyelinase
|
|
|
PKU is a deficiency in?
|
phenylalanine hydroxylase (get accumulation of phenylalanine=neurotoxin)
|
|
|
result of excess phenylalanine?
|
irreversible mental retardation, seizures
|
|
|
describe cretinism
|
congenital hypothyroidism
*see growth retardation, severe MR, deaf, mutism, motor spasticity/rigidity |
|
|
neurological symptoms of Wilson's disease?
|
dystonia, tremor, ataxia
|
|
|
what is the location of CNS injury in wilson's disease?
|
basal ganglia (esp. putamen, caudate nucleus)
|
|
|
define spina bifida occulta
|
vertebral arch defect
|
|
|
describe a meningocele
|
cyst like protrusion of the meninges
|
|
|
describe a meningomyelocele
|
cyst like protrusion of the meninges with nerve roots and spinal cord elements. (neurological problems are common)
|
|
|
describe a rachischisis
|
complete failure of closure of the caudal end of the neuropore
|
|
|
describe anencephaly
|
failed closure of the anterior neuropore
|
|
|
describe Wernicke's encephalopathy
|
acute deficiency of thiamine (B1) - manifests as confusion, opthalmoplegia, altered temperature regulation. FATAL if untreated.
|
|
|
describe Korsakoff's syndrome
|
chronic deficiency of B1 (thiamine) - see antegrade amnesia, confabulation
|
|
|
in which thiamine deficiency are petechial lesions seen in the mammillary bodies?
|
Wernicke's
|
|
|
cardiovascular manifestations of a thiamine deficiency?
|
Beriberi heart disease
|
|
|
neurological manifestations of a B12 deficiency?
|
ataxia
(atrophy of posterior columns - position sense) *sensory > motor deficits, dysesthesias common |
|
|
which portion of the cerebellum is commonly atrophied in chronic alcoholism?
|
cerebellar vermis
|
|
|
besides atrophy of the cerebellar vermis, what other CNS changes are seen in relation to chronic alcoholism?
|
1. cortical atrophy
2. hydrocephalus-ex-vacuo (ventricles look larger due to cortical atrophy) |
|
|
Parkinson's disease generally impairs voluntary or involuntary functions?
|
involuntary functions
|
|
|
what type of tremor is seen in PD?
|
resting tremor
("pill rolling" tremor) |
|
|
avg. age of onset of PD?
|
45-60
|
|
|
PD involves a loss of?
|
DA neurons in the substantia nigra and locus ceruleus
|
|
|
what are Lewy bodies composed of?
|
a-synuclein
|
|
|
what characterizes parkinsonism-dementia complex of Guam?
|
parkinsonism w/ progressive dementia, death in 5-10 yrs
|
|
|
how does progressive supranuclear palsy (PSP) differ from PD?
|
PSP - no Lewy bodies, neuronal loss is in brainstem, basal ganglia, cerebellum
|
|
|
some possible etiologies of parkinsonism? (4)
|
1. post encephalitic
2. drug induced 3. toxin related (MPTP) 4. repeated head trauma |
|
|
inheritance pattern of HD?
|
AD
|
|
|
describe the progression of HD
|
early cognitive and emotional impairment
* then, extrapyramidal symptoms (choreoathetosis) *eventual incapacitation |
|
|
gross pathology of a brain with HD?
|
atrophy of frontal cortex, striatum
* secondary lateral ventricle enlargement |
|
|
HD involves a mutation in a gene on which chromosome?
|
chromosome 4
|
|
|
MC genetic mutation type seen in HD?
|
trinucleotide repeat expansion
|
|
|
ALS involves which neurons?
|
upper and lower MOTOR neurons
|
|
|
describe the clinical course of ALS
|
* progressive muscular weakness
* atrophy, cramps, fasciculations * motor speech disturbance * intellect intact * death usually w/i 3-5 yrs |
|
|
avg. age of onset of ALS?
|
40-60 yrs
|
|
|
gender differences in ALS?
|
M>F
|
|
|
familial ALS (5-10%) is related to a mutation in?
|
superoxide dismutase 1 gene (SOD1)
|
|
|
nervous system areas that atrophy in ALS?
|
* cortical motor neurons
* motor nuclei of brain stem * loss of axons in LCST * anterior horn spinal cord neurons * anterior spinal nerve roots |
|
|
which degenerative disease accounts for >50% of dementia
|
AD
|
|
|
characteristics of AD?
|
progressive dementia with memory and cognitive impairment
|
|
|
risk factors for AD? (3)
|
1. age
2. family history 3. female gender |
|
|
what genetic disorder does AD have a strong connection to?
|
Down's syndrome (trisomy 21)
|
|
|
Four etiological factors that may contribute to AD?
|
1. amyloid beta
2. amyloid precursor protein 3. Apoprotein E e4 4. Presenilins |
|
|
function of presenilins?
|
create amyloid protein from amyloid precursor protein (APP)
|
|
|
mutations in presenilins result in?
|
most cases of familial AD
|
|
|
which type of apolipoprotein has the lowest risk of AD?
|
ApoE e2 or 3
|
|
|
gross characteristics of AD?
|
*cortical atrophy (esp. frontal-parietal, temporal)
*atrophy of gyri, widening of sulci * hydrocephalus ex vacuo |
|
|
microscopic features of AD?
|
*neurofibrillary tangles
*neuritic plaques *granulovascular degeneraton *Hirano bodies |
|
|
in AD, neurofibrillary tangles are primarily made up of what protein?
|
tau (microtubule associated protein)
|
|
|
in AD, what is found at the core of neuritic plaques?
|
amyloid
|
|
|
in AD, where is granulovascular degeneration found?
|
in the cytoplasm of hippocampal neurons
|
|
|
in AD, what are Hirano bodies?
|
eosinophilic bodies by hippocampal neurons (seen alongside granulovascular degeneration)
|
|
|
describe diffuse Lewy body disease
|
dementia with Lewy bodies, may have accompanying akinetic rigidity syndrome (Parkinson like)
|
|
|
gross characteristics of Lewy body dementia?
|
not much, relatively little atrophy
|
|
|
gross features of Pick's disease?
|
*marked cerebral atrophy limited to frontal and temporal lobes
*knife-blade gyri |
|
|
what is a Pick body?
|
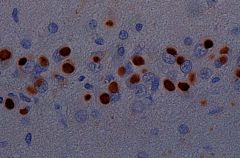
neurons with eosinophilic cytoplasmic inclusions
|
|
|
which stain is used to see Pick bodies?
|
silver stain
|
|
|
50% of dementia can be classified as?
|
AD
|
|
|
dementia in which a personality change or language disorder is seen is called?
|
frontotemporal dementia
(10% of all dementias) |
|
|
what must be present in order to make the diagnosis of dementia?
|
acquired memory impairment WITH impairment in other domains (cognitive or social).
|
|
|
how common is dementia?
|
5-10% of community dwelling adults over the age of 65
50% of those over the age of 80. 70% of all nursing home pts. |
|
|
how can a person have cognitive impairment but not meet the definition of dementia?
|
poor memory performance but NORMAL general cognition and ADLs
|
|
|
what are some "reversible" causes of dementia? (7)
|
1. depression
2. medication 3. alcohol use 4. delerium 5. chronic menigitis 6. tumor 7. normal pressure hydrocephalus |
|
|
describe progressive pseudodementia
|
paitents describe cognitive complaints, have difficulty encoding memories.
DURATION OF DEMENTIA IS SHORTER, depression common, antidepressant trial often warranted |
|
|
List 5 medications commonly responsible for cognitive decline
|
1. pain meds
2. anticholinergics (amitryptiline, nortryptaline) 3. antihypertensives 4. psychotropics 5. sedative-hypnotics (sleeping pills) |
|
|
how common is true inherited AD?
|
<5%
|
|
|
mean survival of an AD patient?
|
8.1 years
|
|
|
MC cause of death in an AD patient?
|
pneumonia
|
|
|
physical manifestations of late AD?
|
increased tone,
myoclonus, seizures, bradykinesia, incontinence, dysphagia, weight loss |
|
|
psychiatric manifestations of AD?
|
depression
delusions hallucinations personality change |
|
|
in which type of dementia are hallucinations very common?
|
Lewy body dementia
|
|
|
6 risk factors for AD?
|
1. Apo-E4-Allele
2. Family history 3. age 4. female 5. head injury 6. lower intelligence/smaller head size |
|
|
two pharmacologic treatments of AD?
|
1. acetylcholinesterase inhibitors
2. NMDA antagonists (Namenda) |
|
|
what is the second leading cause of dementia?
|
Vascular dementia
(single stroke, multi-infarct, subcortical arteriosclerotic encephalopathy) |
|
|
what is it called when vascular dementia is mixed with Alzheimer's pathology?
|
mixed dementia
|
|
|
describe the classic onset of vascular dementia
|
sudden onset or stepwise deterioration
|
|
|
how can vascular dementia be diagnosed?
|
abnormal imaging studies
(also see focal neurological signs and stroke risk factors) |
|
|
what types of strokes cause dementia?
|
lacunar strokes
strategically placed "normal" stroke |
|
|
what does a white matter change indicate?
|
many things, SOMETIMES can indicate dementia and/or AD.
|
|
|
what is the 3rd cause of dementia?
|
subcortical arteriosclerotic encephalopathy (SAE), aka. Binswanger's disease
|
|
|
where is demyelination and gliosis seen in subcortical arteriosclerotic encephalopathy?
|
periventricular
|
|
|
3 psychological findings in SAE?
|
psychomotor retardation
apathy abula (lack of initiative) |
|
|
5 physical findings in SAE?
|
1. pseudobulbar brainstem signs (trouble swallowing)
2. hypperreflexia 3. upgoing toes 4. gait disturbance 5. incontinence |
|
|
treatment for multi-stroke dementia? (4)
|
*antithrombotic
*cholinesterase inhibitors *memantine *supportive, risk factor management |
|
|
avg. age of onset of frontotemporal dementias?
|
EARLY <65
|
|
|
three distinct pathologies of frontotemporal dementia?
|
1. Pick's disease
2. Frontal lobe degeneration 3. progressive subcortical gliosis |
|
|
what is semantic dementia?
|
fluent empty speech "verbal diarrhea"
(loss of knowledge about items, trouble with naming, word comprehension. grammar and pronunciation remains intact) |
|
|
what is progressive nonfluent aphasia?
|
nonfluent speech, progresses to muteness
|
|
|
describe the progression of CJD
|
subacute dementia with myoclonus, rapidly progressive
*80% dead in 1 yr, 50% dead w/i 5 mo. |
|
|
avg age of onset of CJD?
|
50-75 yrs
|
|
|
pathogenesis of CJD?
|
prion protein (PRP - normally an a-helix) changes conformation to a beta-pleated sheet (bad form: protease resistant). this form accumulates in neuronal tissue, causes neuronal death.
|
|
|
7 classical signs/symptoms of Parkinsonism?
|
1. rigidity
2. bradykinesia 3. tremor 4. stooped posture 5. instability 6. changes in handwriting 7. sleep disorders |
|
|
T/F: DBS helps parkinsonism
|
FALSE
DBS only helps PD, not all cases of Parkinsonism |
|
|
PD makes up for what proportion of parkinsonism cases?
|
2/3
|
|
|
5 other causes of parkinsonism?
|
1. PSP (progressive supranuclear palsy)
2. MSA (Multi-system atrophy) 3. CBD (Corticobasal degeneration) 4. LBD (Lewy body disease) 5. stroke others |
|
|
which two races have the lowest incidence of PD?
|
Asians
African Americans |
|
|
what type of tremor is seen in PD?
|
resting
(may be absent in 1/4th of all PD patients) |
|
|
voice changes seen in PD?
|
hypophonia
(soft, tremulous voice) |
|
|
what clues suggest a diagnosis OTHER than PD? (7)
|
1. lack of tremor
2. prominent/early dementia 3. autonomic dysfunction 4. early instability/falls 5. eye movement difficulties 6. lack of response to levodopa 7. early hallucinations |
|
|
how many DA neurons must be lost before motor symptoms appear in PD and a diagnosis is made?
|
80%
|
|
|
how long before the onset of PD does loss of the DA neurons begin?
|
20-30 yrs prior
|
|
|
early symptoms of PD? (5)
|
1. anxiety, depression
2. changes in sense of smell 3. constipation 4. postural changes 5. sleep disorder/weight loss |
|
|
in the on-off phenomenon, what does the off phase correspond to?
|
no DA stimulation
|
|
|
in a PD patient, what are the leading cognitive risk factors for nursing home placement?
|
delerium and dementia
|
|
|
three stages of PD?
|
1. honeymoon period
2. battle area 3. late phase |
|
|
which type of PD is thought to have a better prognosis?
|
tremor predominant PD
|
|
|
which 2 types of PD are thought to have a prolonged course?
|
unilateral disease
young patients |
|
|
gold standard treatment of PD?
|
Sinemet (carbidopa/levodopa)
|
|
|
MOA of carbidopa/levadopa?
|
*levadopa causes peripheral side effects (toxic)
*carbidopa counteracts these peripheral side effects (MAO inhibitor, blocks levodopa breakdown into toxins in periophery) |
|
|
how can nausea be treated in the PD patient on carbidopa/levadopa?
|
increase carbidopa
take with meals |
|
|
3 dopamine agonists used to treat PD?
|
1. Mirapex
2. Permax 3. Bromocriptine |
|
|
neuroprotectant used to prevent progression of PD?
|
Selegeline
|
|
|
role of Entacapone (Comptan) in PD treatment?
|
extends 1/2 life of levadopa
|
|
|
3 anticholinergics used to treat PD?
|
1. Congentin
2. Artane 3. Benadryl |
|
|
MOA of Amantadine?
|
NMDA antagonist
|
|
|
in DBS for PD, where are the electrodes placed (1 of 3 locations)
|
1. globus pallidus
2. subthalamic nucleus 3. thalamus |
|
|
2 positive outcomes of surgery for PD?
|
1. improves time spent in "on" state
2. decreases need for meds |
|
|
risks/negative outcomes of surgery for PD?
|
*risk of bleed, stroke, infx
*risk of masking dementia, dysarthria *poor placement or equipment failure |
|
|
characteristics of Lewy Body Dementia (DLBD) when it comes to meds?
|
very sensitive to meds & their adverse effects - treat gingerly
|
|
|
in which sex is DLBD more common?
|
males
|
|
|
MC of death in DLBD?
|
aspiration pneumonia
|
|
|
how long from symptoms to death in DLBD?
|
6.4 yrs
|
|
|
where are the Lewy Bodies found in DLBD?
|
dopaminergic neurons (diffusely located)
|
|
|
treatment of DLBD?
|
1. low dose carbidopa/levadopa
2. low dose antipsychotic for hallucinations (quetiapine or clozapine) 3. AChE inhibitor for cognition, behavior problems 4. Klonopin for periodic limb movements of sleep |
|
|
hallmark symptom of progressive supranuclear palsy (PSP)?
|
supranuclear opthalmoplegia
|
|
|
does PSP respond to levadopa?
|
NO
|
|
|
avg age of onset of PSP?
|
60s-70s
|
|
|
regarding PSP: on average, how long after diagnosis does death occur?
|
6 yrs
|
|
|
Treatment for PSP? (3)
|
1. Sinemet - limited motor response
2. SSRIs (depression/anxiety, pseudobulbar effect) 3. AChEIs for dementia |
|
|
3 variants of multiple system atrophy (MSA)?
|
1. Shy Drager syndrome (MSA-A)
2. striatonigral degeneration (MSA-B) 3. OPCA (MSA-C) |
|
|
characteristics of MSA-A (Shy-Drager syndrome)?
|
dysautonomia (faining, cold hands, mottling, incontinence)
|
|
|
characteristics of striatonigral degeneration (MSA-B)?
|
Parkinsonian symptoms
|
|
|
characteristics of OPCA (MSA-C)
|
ataxia
|
|
|
treatment for MSA?
|
PT/Speech Therapy
early response to levadopa fludrocortisone or midodrine for BP support |
|
|
four causes of acquired ataxias?
|
1. alcohol
2. chemotherapy 3. infections 4. paraneoplastic |
|
|
two inherited ataxias?
|
1. Friedreich's ataxia (AR)
2. Spinocerebellar ataxia (AD and sporatic) |
|
|
what is the rarest Parkinsonian condition?
|
Corticobasal degeneration
|
|
|
age of onset of corticobasal degeneration?
|
60's or later
|
|
|
s/s of corticobasal degeneration?
|
"alien limb phenomenon"
asymmetric parkinsonism and limb dystonia WITH UMN signs and late dementia |
|
|
"lower-half" parkinsonism with prominent gait disturbance, subcortical dementia, AND a history of stroke is called?
|
vascular parkinsonism
|
|
|
what is the cause of dementia pugulistica?
|
repeated head trauma
|
|
|
s/s of dementia pugilistica?
|
*attentional and memory deficits
*parkinsonism |
|
|
is there any genetic group that is at higher risk of dementia pugilistica?
|
ApoE4 carriers
|
|
|
symptomatic, communicating, adult onset hydrocephalus is known as?
|
normal pressure hydrocephalus
|
|
|
what is the triad seen in normal pressure hydrocephalus?
|
1. subcortical dementia (subacute)
2. frontal "magnetic" gait disturbance 3. urinary incontinence |
|
|
what is frequently seen in normal pressure hydrocephalus?
|
parkinsonism
(with bradykinesia, rigidity, limb tremors) |
|
|
causes of normal pressure hydrocephalus? (3)
|
1. remote trauma, infection
2. subarachnoid hemorrhage 3. idiopathic |
|
|
of the triad seen in normal pressure hydrocephalus, which symptom is earliest?
|
gait disturbance (apraxic gait - "stuck to floor" feeling)
|
|
|
MRI findings in NPH?
|
1. ventriculomegaly
2. thinning of corpus callosum 3. no CSF flow |
|
|
shunting is what is normally done to treat normal pressure hydrocephalus. when is it less effective?
|
if small vessel disease is present
|
|
|
success rates/results of shunting in NPH?
|
1/3 have marked improvement
1/4 have mild improvement 1/4 have moderate complications 1/4 have shunt malfunction |
|
|
describe an essential tremor
|
tremor that is most promiment with intention.
*often distal in the limb |
|
|
which type of tremor, resting or essential, is responsive to alcohol?
|
essential
|
|
|
what am I?
hyperkinetic movements with irregular, unpredictable, brief, jerky movements that move from one part of the body to another... |
chorea
|
|
|
what is the difference between chorea and choreoathetosis?
|
choreoathetosis is slower than the characteristically brisk chorea
|
|
|
what am I?
inherited, progressive disorder manifested by chorea and other movements, behavior disorder and dementia |
HD
|
|
|
inheritence pattern of HD?
|
AD
|
|
|
when do s/s of HD appear?
|
in the 4th or 5th decade
|
|
|
where is the HD gene and what happens genetically in HD?
|
chromosome 4
HD is the result of an expanded CAG trinucleotide sequence |
|
|
genetic correlation between severity of the disease?
|
increased number of repeats = increased severity of disease
|
|
|
under which type of inheritance is the number of repeats in HD more likely to increase?
|
father to child
|
|
|
what type of reflexes would most likely be present in a HD patient?
|
brisk reflexes
upgoing toes |
|
|
what part of the brain atrophies in HD?
|
caudate
|
|
|
what is the life expectancy after an HD diagnosis?
|
10-20 yrs
|
|
|
MCC of death in HD?
|
aspiration
|
|
|
signifance of earlier onset HD as compared with "normal" onset?
|
earlier onset has faster progression and seizures are more likely
|
|
|
the condition of chronic dopamine receptor blockade is known as?
|
tardive dyskinesia
|
|
|
MC location of tardive dyskinesia?
|
around the face
(can occur in the body as well) |
|
|
what disorder am I?
disorder of sustained muscle contraction which creates abnormal posture? |
dystonia
|
|
|
generalized dystonia is often inherited. it is due to a mutation of?
|
the DYT1 gene
|
|
|
any treatment for dystonia?
|
dopamine
SOME are dopamine responsive, others are not |
|
|
which type of dystonia often starts late in adult life?
|
focal or segmental
|
|
|
which sex is tics more common in?
|
males
|
|
|
age of onset of tics?
|
childhood
|
|
|
what is Tourette's syndrome?
|
childhood onset of motor and vocal tics
|
|
|
inheritance pattern of Tourette's syndrome (TS)?
|
AD
|
|
|
TS is commonly seen with what other conduct disorders? (2)
|
ADHD
OCD |
|
|
which disorder am I?
sudden, brief, shock-like involuntary movements |
myoclonus
|
|
|
myoclonus is seen in what brain diseases/injuries? (3)
|
1. hypoxic brain injury
2. late stage neurodegenerative diseases 3. early stage CJD |
|
|
name three metabolic movement disorders with associated dementia
|
1. Wilsons disease
2. Hallevorden-Spatz 2. Idiopathic basal ganglia calcification |
|
|
what is Hallevorden-Spatz disease?
|
early onset dementia with parkinsonism - iron deposition in basal ganglia
|
|
|
idiopathic basal ganglia calcification presents as?
|
dementia with parkinsonism
(familial disorder) |
|
|
inheritance pattern of Wilsons disease?
|
AR
|
|
|
when does Wilson's disease present?
|
2nd and 3rd decade
|
|
|
s/s of Wilson's disease?
|
*parkinsonism
*cerebellar signs *limb tremors: "wing beating" *cognitive abnormalities *Kayser-Fleischer rings |
|
|
imaging abnormalities in Wilsons disease?
|
copper deposition in deep brain on MRI
|
|
|
define pachymeningitis
|
an inflammation of the dura
(abundant pus present, thick green exudate) |
|
|
two MC organisms to cause pachymeningitis?
|
Staph or Strep
|
|
|
source of the bacteria that cause pachymeningitis?
|
1. skull defect (fracture)
2. infection of paranasal sinuses |
|
|
define leptomeningitis
|
inflammation of the meninges (esp. arachnoid tissue and subarachnoid space)
|
|
|
3 MC causative organisms of leptomeningitis?
|
1. Strep. penumoniae
2. N. meningitidis 3. Group B Strep |
|
|
MC causative agent of neonatal meningitis?
|
Group B Strep (~50)
e. coli is a close 2nd with 20% |
|
|
what is the only spirochete infection of the brain?
|
T. pallidum
syphilis |
|
|
which bacteria MC causes brain abscesses?
|
Staph aureus
|
|
|
which 2 fungi MC cause brain abscesses?
|
Aspergillus
Mucor (lid pushers) |
|
|
causative agent of leprosy?
|
Mycobacterium leprae
|
|
|
leprosy infects what part of the nervous system?
|
Schwann cells (PNS)
|
|
|
fatality rate of meningitis?
|
HIGH (70% if untreated)
|
|
|
progression of a typical meningitis case?
|
death within 24 hrs
|
|
|
complications of meningitis?
|
slow treatment can lead to deafness, blindness, mental deficiency (10-20%)
|
|
|
classic presentation of meningitis?
|
HA
stiff neck vomiting fever confusion delirium |
|
|
how can meningitis be differentiated from septicemia?
|
meningitis: severe HA, stiff neck, pain on moving neck
septicemia: rash, cold hands and feet, rapid breathing, pain in muscles, joints, abdomen |
|
|
describe the rash seen in septicemia
|
petechial rash
(push and doesn't go away) |
|
|
what are the NEW sepsis warning signs Dr. Young presented?
|
leg pain
cold hands and feet abnormal skin color (pallor, mottling) *see pt. again in 4-6 hrs if concerned about condition |
|
|
what is Kernig sign in relation to a diagnosis of meningitis?
|
patient cannot or will not straighten legs because the pain is too great. knees remain up by chin.
|
|
|
what is the Brudzinski's sign in relationship to diagnosis of meningitis?
|
attempt to raise patients head when they are lying flat. patient involuntarily raises hips and knees to relieve pain.
|
|
|
describe the following lab studies in a patient with meningitis
1. gram stain 2. immune cells 3. protein levels 4. glucose levels |
1. + for bacteria and fungi
2. increased (host response to bacteria) 3. increased (due to large # of bacteria) 4. decreased (glc. is being metabolized by bacteria) |
|
|
treatment for meningitis? (4)
|
1. control intracranial pressure (anti-inflammatory agents, osmotic agents, shunt)
2. adequate oxygenation 3. monitor blood glucose 4. broad spectrum antibiotic |
|
|
transmission of meningitis?
|
direct contact (droplet infection)
|
|
|
colonization and spread of bacteria causing meningitis?
|
colonize in mucosa of upper respiratory tract. spread to skin, eyes, heart, adrenal glands, meninges
|
|
|
an important virulence factor a bacteria must have in order to cause meningitis is the ability to cross the BBB. Via what 3 methods is this accomplished?
|
1. transcellular
2. paracellular 3. trojan horse (via phagocytes) |
|
|
what mediates the neural damage seen in/after meningitis?
|
inflammation
|
|
|
the MC cause of meningitis used to be ? and now is ?
|
used to be H. influenzae
now is S. pneumoniae |
|
|
reason for the change in major causative organism of meningitis?
|
H. influenzae type b (Hib) vaccine.
|
|
|
changes in the age of meningitis patients?
|
mean age is now 25 yrs. (used to be 15 months)
|
|
|
H. influenzae is what type of bacteria?
|
gram - coccobacillus
facultative anaerobe |
|
|
MC antigenic type of H. influenzae?
|
type a
used to be type b (not anymore due to Hib vaccine) |
|
|
is H. influenzae fastidious?
|
yes, it needs chocolate agar to grow (requires hemin and NAD)
|
|
|
is H. influenzae a hardy bacteria?
|
NO!
very sensitive to drying, hot and cold, disinfectants |
|
|
reservoir of H. influenzae?
|
human nasopharynx
(esp. in crowding) |
|
|
5 virulence factors of H. influenzae?
|
1. capsule
2. pili 3. IgA protease 4. endotoxin (LPS) 5. phase variation |
|
|
how much phase variation does H. influenzae have?
|
LOTS - has more phase variation than any other known pathogen!
|
|
|
describe epiglottitis
|
caused by H.influenzae. life threatening emergency: epiglottis swells to 5-10 times normal size and becomes bright red. obstructs airway, death due to asphyxiation.
|
|
|
causative agent of regular old pink eye?
|
H. aegypticus (or Strep)
|
|
|
what is Brazilian hemorrhagic fever?
|
"pink eye on steroids"
caused by a more virulent strain of H.aegypticus that invades and grows in the bloodstream. |
|
|
what is the best treatment for an H.influenzae infection?
|
new B-lactams, chloramphenicol or tetracycline
*also treat family members to eradicate carriers* |
|
|
describe the type and shape of Neisseria meningitidis
|
Gram - coccus
(kidney bean shaped diplococci) |
|
|
is Neisseria meningitidis oxidase + or -?
|
oxidase +
|
|
|
reservoir of Neisseria meningitidis?
|
human nasopharynx
(higher in crowded populations) |
|
|
5 virulence factors of Neisseria meningitidis?
|
1. adhesins
2. pabulins (siderophores) 3. evasins (protease and polysaccharide) 4. toxins (LPS) 5. phage (associated with high virulence) |
|
|
where are epidemics of Neisseria meningitidis meningitis commonly seen?
|
crowded areas: military bases, dormitories
|
|
|
3 gross changes seen in meningitis caused by Neisseria meningitidis?
|
1. congested vessels
2. small perivascular hemorrhages 3. no obvious meningitis in brain |
|
|
which strain of Neisseria meningitidis is known to have increased virulence?
|
Type C strain
|
|
|
define fulminant meningitis caused by Neisseria meningitidis
|
septicemia that appears so fast meningitis has no time to establish
|
|
|
for which strain of Neisseria meningitidis is there a vaccine?
|
Type C
|
|
|
petechial hemorrhages on the body indicate that the meningitis has progressed to?
|
septicemia
|
|
|
manifestations of septicemia in the adrenal glands?
|
hemorrhagic necrosis
(Waterhouse-Friederichsen syndrome) |
|
|
commonly seen cardiac manifestation of septicemia?
|
acute myocarditis
|
|
|
pathogenesis of petechiae formation seen in septicemia?
|
thrombosis
(loss of activated protein C leads to loss of anti-coagulation) |
|
|
Treatment of DIC mediated septicemia?
|
activated protein C is the only drug approved for severe sepsis
|
|
|
what happens if unactivated protein C is administered in the treatment of septicemia?
|
makes clotting worse
|
|
|
two causative agents of subacute or chronic meningitis?
|
1. Mycobacterium tuberculosis
2. cryptococcus neoformans (yeast) |
|
|
manifestations of mycobacterium tuberculosis in the brain?
|
caseated granulomas in the ventricular system
|
|
|
manifestations of C.neoformans in the brain?
|
minimal inflammatory response
see a creamy gelatinous exudate concentrated over the base of the brain |
|
|
how is Group B Strep transmitted to the neonate to cause neonatal meningitis?
|
acquired from the mother during birth
(treat prophylactically in 3rd trimester) |
|
|
role of Beta-hemolysin /cytolysin in the pathogenesis of neonatal meningitis?
|
*initial entrance into neonate lungs.
*immune evasion (kills PMNs and macrophages). *enables Crossing of the BBB and initiates inflammatory response in the brain. |
|
|
two variations in clinical presentation of leprosy?
|
1. Tuberculoid leprosy (localized)
2. Lepromatous leprosy (Hansen's disease) |
|
|
how is tuberculoid leprosy localized?
|
via cell cell mediated immunity: see development of macules (large flattened patches)
|
|
|
which type of leprosy is not self limiting?
|
Lepromatous leprosy
(Tuberculoid leprosy lasts ~18 yrs) |
|
|
why is lepromatous leprosy a disseminated infection?
|
there is defective cellular immunity
|
|
|
in which type of leprosy will acid fast bacilli be seen in tissue biopsy/skin smears?
|
lepromatous leprosy
|
|
|
4 s/s of lepromatous leprosy?
|
1. loss of feeling (results in burns and scars)
2. thickening of nerves 3. lion face (loss of eyebrows, deformed nostrils, thick or lumpy earlobes) 4. marks or rings without feeling inside |
|
|
what is the difference between paucibacillary and multibacillary leprosy?
|
paucobacillary - 5 or less skin lesions, no bacilli present in biopsy/smear
multibacillary - 6 or more skin lesions, bacilli may be present in biopsy/smear |
|
|
3 drugs used to treat leprosy?
|
1. sulfones (ie. dapsone)
2. rifampin 3. clofazimine |
|
|
MOA of sulfones in the treatment of leprosy?
|
inhibits PABA, reduces transmission
|
|
|
MOA of rifampin in the treatment of leprosy?
|
inhibits RNA polymerase (bacterial transcription).
patients become non-infectious in 2 days |
|
|
treatment regimen for paucibacillary leprosy?
|
dapsone
rifampin *both daily for 1 yr* |
|
|
treatment regimen for multibacillary leprosy?
|
dapsone
rifampin clofazimine *all daily for 2 yrs* |
|
|
3 drugs used to treat leprosy?
|
1. sulfones (ie. dapsone)
2. rifampin 3. clofazimine |
|
|
MOA of sulfones in the treatment of leprosy?
|
inhibits PABA, reduces transmission
|
|
|
MOA of rifampin in the treatment of leprosy?
|
inhibits RNA polymerase (bacterial transcription).
patients become non-infectious in 2 days |
|
|
treatment regimen for paucibacillary leprosy?
|
dapsone
rifampin *both daily for 1 yr* |
|
|
treatment regimen for multibacillary leprosy?
|
dapsone
rifampin clofazimine *all daily for 2 yrs* |
|
|
maintenance regimens for patients with leprosy?
|
daily dapsone or clofazimine for life!
|
|
|
is Mycobacterium leprae motile?
|
no
|
|
|
describe the growth pattern of Mycobacterium leprae.
|
intracellular
*cannot be cultivated in vitro* |
|
|
reservoir of Mycobacterium leprae?
|
humans
armadillos (can grow organism on nude mouse) |
|
|
the genome of Mycobacterium leprae is very close to the genome of?
|
Mycobacterium tuberculosis
|
|
|
is there variation between the types of Mycobacterium leprae that cause leprosy?
|
NO!
one clone has infected the entire world! |
|
|
pathogenesis of Mycobacterium leprae?
|
bacteria attach to and invade Schwann cells of PNS (only pathogen known to do so, only infects Schwann cells that are not yet making myelin)
|
|
|
Where (genetically) is the host susceptibility locus for leprosy?
|
short arm of chromosome 10
|
|
|
3 goals for antiepileptic agents?
|
1. efficacious
2. safe 3. no interference with quality of life |
|
|
10 seizure triggers?
|
1. trauma
2. illness 3. fever 4. hyperventilation 5. alkalosis 6. loss of sleep 7. hormonal changes 8. fluid and electrolyte imbalances 9. etoh, drugs 10. w/d from meds |
|
|
6 factors in achieving seizure control?
|
1. adequate meds
2. adequate sleep, rest, good nutrition 3. fluid and electrolyte balance 4. acidosis 5. low anxiety level 6. etoh abstinence |
|
|
mechanism behind anxiety causing seizures?
|
increased catecholamines precipitates seizures
|
|
|
mechanism behind etoh lowering seizure threshold?
|
etoh is a depressant. it causes compensation by increasing excitation factors -> this lowers seizure threshold.
|
|
|
which female hormone is protective against seizures?
|
progesterone
|
|
|
effect of hyperventilation on seizure threshold?
|
lowers threshold, often precipitates them
(causes alkalosis) |
|
|
what are the two historic features most suggestive of a seizure?
|
1. aura
2. post-ictal confusional state |
|
|
what are some metabolic/electrolyte derangements that can precipitate seizures? (7)
|
1. hypoglycemia
2. hyponatremia 3. hyperosmolar states 4. hypocalcemia 5. uremia 6. hepatic encephalopathy 7. porphyria |
|
|
sudden withdrawal of what well known drugs can precipate seizures? (8)
|
1. etoh
2. antidepressants 3. antipsychotics 4. insulin 5. isoniazid 6. lidocaine 7. methylxanthines 8. cocaine |
|
|
primary neurologic disorders that manifest as seizures? (6)
|
1. benign febrile convulsions
2. idiopathic epilepsy 3. head trauma 4. stroke 5. mass lesions (tumor, abscess) 6. infection (meningitis, encephalitis) |
|
|
four components of a seizure?
|
1. aura
2. ictal 3. postictal 4. interictal |
|
|
what does the aura of a seizure represent?
|
represents the beginning of the attack (paroxysmal neuron firing in or near the focus)
|
|
|
what defines the interictal period of a seizure?
|
period between the attacks (clinically normal)
|
|
|
hyperventilation may precipitate what kind of seizure?
|
absence seizures
|
|
|
what is catamenial epilepsy?
|
most seizures occur during menstruation
|
|
|
what concentrations of AEDs may cause seizures?
|
too much or too little
|
|
|
what are the three most clearly established risk factors for epilepsy?
|
1. severe head trauma
2. CNS infections 3. stroke |
|
|
what is the main difference between a simple and complex partial seizure?
|
simple - consciousness not impaired
complex - consciousness impaired |
|
|
characteristics of viral encephalitis?
|
1. encephalopathy (consciousness change)
2. fever 3. seizures 4. focal neurologic findings 5. abnormal EEG 6. abnormal neuroimaging |
|
|
define aseptic meningitis
|
meningitis where no bacteria can be found in the CSF
*assume a viral cause* |
|
|
what is the MCC of acute, sporadic viral encephalitis in the US?
|
herpes simplex virus
|
|
|
herpes encephalitis tends to involve which area of the brain?
|
orbitofrontal region and temporal lobe
|
|
|
in regards to HSV, what are the major routes of access to the CNS?
|
olfactory, trigeminal nerves
|
|
|
describe the progression of herpes encephalitis
|
starts as flu-like symptoms
followed by abrupt onset of neurological symptoms |
|
|
what are the neurological symptoms seen in herpes encephalitis?
|
severe H/A
altered behavior decreasing LOC focal neurologic findings convulsions/seizures |
|
|
what is the key lab value in distinguishing viral from bacterial encephalitis?
|
CSF glucose levels
(low in bacterial, normal in viral) |
|
|
how is HSV determined as the cause of encephalitis?
|
PCR
(can also look for viral antigens in CSF by immunoassay, but can only detect 5 days after onset, whereas PCR can detect 1-2 days after onset) |
|
|
characteristics histological findings in herpes encephalitis?
|
Chowdry A inclusions
(eosinophilic center with dark chromatin at margin of nucleus) |
|
|
treatment of herpes encephalitis?
|
acyclovir
|
|
|
what other herpesviruses cause CNS infections?
|
1. CMV (encephalitis)
2. VZV (encephalitis) 3. HSV (aseptic meningitis) |
|
|
CMV encephalitis is MC seen in ?
|
immunocompromised patients
fetuses |
|
|
risk factors for VZV encephalitis?
|
advanced age
CN involvement |
|
|
what is the polio eradication success attributed to?
|
1. limited # of serotypes (3)
2. humans are the sole viral reservoir |
|
|
poliovirus:
1. is what kind of virus? 2. belongs to which family? |
1. enterovirus
2. picornaviridae |
|
|
describe the genome of poliovirus
|
non-segmented
(+)ssRNA no envelope |
|
|
of the 3 serotypes of poliovirus, which ones are neurotropic and neurovirulent?
|
ALL
|
|
|
transmission of poliovirus?
|
fecal-oral
|
|
|
where does the poliovirus replicate?
|
peyer's patches of the intestinal lamina propria
|
|
|
how does the poliovirus establish infection in other tissues, including the CNS?
|
viremia
|
|
|
once established in the CNS, where does the poliovirus replicate? significance?
|
replicates in the motor neurons of the anterior horns of the spinal cord
*this causes paralysis |
|
|
in relation to a CNS poliovirus infection: paralysis is often preceded by?
|
severe myalgia, then weakness
|
|
|
paralysis affects:
1. which muscles 2. which limbs |
1. proximal > distal
2. legs > arms |
|
|
consequence of poliovirus involving the cervical nerves?
|
can lead to paralysis of the diaphragm and respiratory failure (then need iron lung)
|
|
|
two vaccines that are available for polio?
|
live oral polio vaccine (OPV)
inactivated polio vaccine (IPV) |
|
|
which vaccine is currently recommended for children in the US? why?
|
IPV
(OPV causes vaccine associated paralytic poliomyelitis) |
|
|
aseptic meningitis is the MC type of meningitis in the US. which age group is it MC seen in?
|
neonates (severe)
|
|
|
enteroviral meningitis seen beyond which age is rarely associated with severe disease or a poor outcome?
|
2 wks.
|
|
|
s/s of enteroviral meningitis?
|
sudden onset
fever, nuchal rigidity H/A photophobia |
|
|
which is least severe: enterovial aseptic meningitis or enteroviral encephalitis?
|
enteroviral aseptic meningitis
|
|
|
enteroviral encephalitis may progress to?
|
coma or generalized seizures
paralysis |
|
|
the JC virus causes what CNS disease?
|
Progressive multifocal leukoencephalopathy (PML)
(rare, fatal, demyelinating disease) |
|
|
PML caused by the JC virus is associated with what host status?
|
immunosuppression
|
|
|
following onset, PML usually causes death in how long?
|
3-6 mo
|
|
|
the JC virus targets which CNS cell?
|
oligodendrocytes
(this leads to demyelination) |
|
|
where does the demyelination occur in PML?
|
junction of grey and white cortical matter
|
|
|
in PML: describe the CSF and inflammatory reaction
|
CSF is normal
inflammatory rxn is minimal |
|
|
chronic infection with the measles virus may lead to what CNS disease?
|
subacute sclerosing panencephalitis (SSPE)
|
|
|
what type of patients are at greatest risk for SSPE?
|
children who had measles at <2 yrs of age
|
|
|
prognosis of SSPE?
|
usually fatal
|
|
|
lymphocytic choriomeningitis (LCM) is what kind of a viral disease?
|
rodent-borne
|
|
|
what is the causative agent of lymphocytic choriomeningitis (LCM)?
|
lymphocytic choriomeningitis virus (LCMV)
|
|
|
what is the primary host of lymphocytic choriomeningitis (LCM) and how does infection occur?
|
common house mouse
(exposure to fresh urine, droppings, saliva, nesting materials) |
|
|
prognosis of lymphocytic choriomeningitis (LCM)?
|
benign course
low mortality (may be fatal in immunosuppressed, ie. organ transplant recipients) |

