![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
781 Cards in this Set
- Front
- Back
|
What characterizes Neisseria meningitidis?
|
Gram(-) diplococci, non-motile, non-hemolytic, non-spore forming, capsules, pili;
facultative anaerobes, grow on chocolate agar/Thayer-Martin media; 13 serotypes based on polysaccharide capsule (A, B, C, W-135, Y) |
|
|
What signs/symptoms are associated with N. meningitidis infection?
|
Fever, stiff neck, sore throat, intense HA, photophobia, vomiting, seizures, petechial-purpuric rash – in neonates: temperature instability, listless, weak, v/d, changes in alertness
|
|
|
What are the virulence factors of N. meningitidis?
|
Pili (adherence), IgA1 protease (cleaves host IgA), capsule (resist phagocytosis and complement), meningobactin (iron binding protein), LPS (inflammation)
|
|
|
What is the pathway of infection of N. meningitidis infection?
|
Nasopharyngeal colonization --> invasion of the epithelium --> invasion of blood --> further dissemination (e.g. CSF)
|
|
|
What describes the pathogenesis of N. meningitidis infection?
|
Humans are the sole host, they can be normal flora/pathogen with 8-25% carrier rate (carriers have protective antibodies); Bacteria adhere and invade through the nasopharynx leading to bacteremia, endotoxemia, meningitis; The pathology is, in part, the result of the host inflammatory response
|
|
|
What characterizes the transmission of N. meningitidis?
|
Respiratory droplets, requires close contact
|
|
|
What describes the epidemiology of N. meningitidis infection?
|
>3000/year in US; major serogroups incl. B and C, secondary incl. Y and W-135; isolated cases or small outbreaks in day care centers or with college students; Predisposing factors: complement deficiency, immunocompromised, head trauma, neurodiagnostic procedures, surgery
|
|
|
How does the anatomical location of a CNS infection determine the symptomatology?
|
Frontal lobe abscess: frontal sinusitis, HA, memory defects, attention loss, diminished intellectual performance;
Temporal lobe encephalitis: personality changes, visual field defects, hemiparesis with large lesions, focal seizures; Basilar meningitis: HA (suboccipital), neck stiffness, diplopia, cranial nerve palsies |
|
|
What are common causes of purulent meningitis?
|
Newborns: GBS, E. coli, L. monocytogenes;
Infants/Children: S. pneumo, N. meningitidis, H. flu; Adults: S. pneumo, N. meningitidis, H. flu (rarely) |
|
|
What are the various types of meningitis?
|
Purulent: acute infections, rapid onset, bacterial origin;
Chronic: develop slowly, mycobacteria or fungal origin, Aseptic: self-limiting, viral origin |
|
|
What are the complications of bacterial meningitis?
|
In 10-20%: deafness, mental retardation, seizures, strokes, limb amputations;
10-30% mortality overall (10% mortality in treated patients) |
|
|
What virulence factors are seen with L. monocytogenes?
|
Intracellular pathogen: cell-mediated immunity is protective, humoral response poor;
Hemolysin: pore-forming cytolysin, lyses phagosomal membrane to allow escape from the phagosome, necessary for virulence; ActA: required for actin polymerization and motility |
|
|
What characterizes Listeria monocytogenes?
|
Gram(+) rod, catalase-positive, hemolysin, motile, 13 serotypes (cell wall/flagellar antigens);
Epidemiology: contaminated food, non-pasteurized dairy products, can grow at cold temperatures, can have long incubation period (2-8 wks) |
|
|
What characterizes E. coli?
|
Common cause of neonatal meningitis (resembles disease caused by GBS);
75% of cases are caused by strains with K1 capsular polysaccharide (contains sialic acid, identical to group B N. meningitidis); infection occurs during childbirth from vaginal E. coli, infants are very susceptible – treatment: 3rd generation cephalosporins |
|
|
Which populations most typically receive the MCV4 vaccine?
|
US military recruits, people who might be affected during an outbreak, anyone traveling to commonly affected areas (e.g. W. Africa), anyone with a complement deficiency, college students, adolescents at 11-12yo, laboratory workers routinely exposed to N. meningitidis
|
|
|
What vaccines are available against N. meningitidis?
|
Meningococcal conjugate polysaccharide-protein vaccine (MCV4): for groups A, C, Y, W-135 (given between 11-55yrs, but especially at 11-12 years since this is when social activities start);
Meningococcal polysaccharide vaccine (MPSV4): for groups A, C, Y, W-135 (given >2yrs, for high risk groups like complement deficiencies and asplenia) |
|
|
What is the treatment for bacterial meningitis?
|
Medical emergency: antibiotics that penetrate the meninges/enter CSF (β-lactams, like Cefotaxime), anti-inflammatory drugs (Dexamethasone); Prophylactic antibiotics in contacts of patients: Rifampin, quinolone antibiotics, Ceftriaxone; Antibiotic treatment of carriers
|
|
|
What are the clinical findings with N. meningitidis infection?
|
Acute bacterial meningitis: CSF analysis shows ↑PMNs, ↓glucose, ↑protein, Gram(-) diplococci;
Besides the CSF, organisms can be cultures from blood or a skin lesion; also PCR can be used |
|
|
What does the CSF show in subacute meningitis (mycobacterial/fungal)?
|
↑ leukocytes/PMNs/protein (but even less so than with aseptic viral meningitis), ↓glucose
|
|
|
What does the CSF show in aseptic viral meningitis?
|
↑ leukocytes/PMNs/protein (but less than with bacterial meningitis), normal glucose
|
|
|
What characterizes the symptomatology of L. monocytogenes infection?
|
Normally asymptomatic; can see bacteremia, pneumonia, meningitis;
Pregnant women: miscarriage, granulomatosis infantiseptica; Elderly: more susceptible |
|
|
What describes the diagnosis and treatment of L. monocytogenes infection?
|
Diagnosis: culture organism from blood/CSF/lesion;
Treatment: Penicillin G, Ampicillin, Erythromycin, Chloramphenicol |
|
|
What characterizes poliovirus?
|
ssRNA, (+)sense, infects primates only, non-enveloped, 3 antigenic serotypes
|
|
|
What characterizes the spread of enterovirus inside the body?
|
Initial replication occurs in the intestinal tract in epithelial and lymphoid cells --> virus invades deeper tissues and enters the blood stream causing viremia --> virus may spread to the brain and spinal cord from the blood or by the retrograde axonal route in peripheral/cranial nerves
|
|
|
What characterizes the transmission of enteroviruses?
|
Excreted in large amounts in stool --> fecal-oral route of contamination; outbreaks in temperate climates mainly occur during the summer months, and all year-round in the tropics
|
|
|
What happens when poliovirus particles are released from the host cell?
|
They cause lysis (i.e. it’s a lytic virus)
|
|
|
What characterizes translation and processing of poliovirus?
|
Genome is translated into a single polypeptide (a “polyprotein”) --> polyprotein is processes sequentially by two viral proteases into its structural proteins (VP1-4) and non-structural proteins (e.g. VPg, RNA-dependent RNA polymerase, two proteases)
|
|
|
Which step in the poliovirus life cycle determines the tropism of the virus?
|
Not the receptor binding (since PVR is found in multiple regions, yet only has CNS effects), but some step downstream
|
|
|
What characterizes the replication of poliovirus?
|
Poliovirus attaches to a cellular receptor (PVR or CD155), which is a glycosylated protein at the “canyon” site --> RNA is released to the cytoplasm --> (+)sense RNA serves directly as an mRNA template for translation by host factors --> RNA is linked at the 5’ end to viral protein VPg that is a primer for RNA-dependent RNA polymerase
|
|
|
What characterizes the various types of viral CNS infections?
|
Encephalitis: infection of brain parenchyma;
Meningitis: infection of meninges; Myelitis: infection of spinal cord |
|
|
Which Picornavirus genera are pathogenic to humans?
|
Enterovirus: replicates in GI tract (e.g. polio);
Hepatovirus: cause viral hepatis (e.g. HAV); Parechovirus: enteric cytopathic human orphan virus; Rhinovirus: common cause of cold |
|
|
What characterizes the Picornaviridae?
|
Contain some of the smallest viruses known to infect man (thus pico); virions are icosahedral and about 30nm in diameter
|
|
|
How is the diagnosis of polioviral infection made?
|
Symptoms are non-specific; aseptic meningitis suggests enteroviral etiology; virus can be isolated and cultured from clinical samples (stool, throat, CSF); virus can be detected by ELISA and PCR
|
|
|
What are Rhabdovirus features?
|
They are bullet-shaped and relatively large, enveloped, contain (-)sense RNA
|
|
|
What characterizes Rhabdoviruses?
|
Consists of two genera:
Lyssavirus (e.g. rabies and rabies-related viruses), Vesiculovirus (e.g. vesicular stomatitis virus) – rabies virus infects numerous mammalian species; it is an acute, progressive, incurable encephalitis |
|
|
What is the most frequent cause of enterovirus infection in the US?
|
Coxsackieviruses
|
|
|
Which vaccine should be administered to immune-deficient individuals?
|
Salk vaccine, since it cannot cause poliomyelitis
|
|
|
What two vaccines were made for poliovirus?
|
Salk vaccine: killed vaccine, effective; Sabin vaccine: live, attenuated vaccine, more effective (since it infects the intestine and elicits IgA production, resembling the natural process)
|
|
|
How is the diagnosis of polioviral infection made?
|
Symptoms are non-specific; aseptic meningitis suggests enteroviral etiology; virus can be isolated and cultured from clinical samples (stool, throat, CSF); virus can be detected by ELISA and PCR
|
|
|
What is the treatment for poliovirus infection?
|
No drug therapy currently available; vaccination is the best method
|
|
|
What are Rhabdovirus features?
|
They are bullet-shaped and relatively large, enveloped, contain (-)sense RNA
|
|
|
What describes the time course of poliovirus spread?
|
Incubation period (1-3days): virus replicates in intestine with no or minor symptoms of fever/HA;
Abortive stage (2-6days): virus continues to replicate and initial Abs appear; CNS invasion (5-10 days): virus spreads to CNS and causes paralysis |
|
|
What characterizes the epidemiology of rabies virus?
|
In Africa, Asia, and Latin America, >90% of cases are caused by domestic dog bites, whereas in the US, Canada, and Europe, cases are caused by wild animals (bats, raccoons, foxes, skunks)
|
|
|
What characterizes the post-exposure prophylaxis of humans?
|
Passive administration of Abs: human rabies immune globulin (collected from immunized persons) – Vaccine is prepared from avian embryos or from various human/monkey cell lines (followed by inactivation)
|
|
|
What characterizes the prevention of rabies infection?
|
Since it is a zoonosis, vaccination of pets is mandatory; vaccination of wild animals is also used by spreading baited oral vaccine – pre-exposure prophylaxis is used among high-risk workers; post-exposure prophylaxis includes local wound treatment, administration of Abs, vaccination
|
|
|
How is the diagnosis of rabies infection made?
|
Through a direct fluorescent antibody test (dFA); test is performed on brain impressions (of an animal) with a FITC- conjugated anti-virus antibody; test can also be done with human skin biopsies from neck containing hair follicles surrounded by a nerve network
|
|
|
What symptoms are associated with rabies infection?
|
Early symptoms are non-specific; as the disease progresses, neurological symptoms appear (e.g. insomnia, anxiety, confusion, partial paralysis, excitation, hallucinations, agitation, hypersalivation, difficulty swallowing, and hydrophobia); eventually patients progress to coma and die within days of the onset of symptoms
|
|
|
What characterizes the spread and pathology of rabies virus?
|
Virus infects at the bite site and replicates --> incubation period is 1-3 months (can be a year or longer) --> moves to sensory neurons and travels towards the CNS --> within the CNS it causes encephalitis --> neurons die and demyelination occurs resulting in encephalopathy --> virus often returns to the periphery with preference to salivary glands
|
|
|
What happens with rabies virus once it gains access to an animal (e.g. raccoons)?
|
The virus moves along peripheral nerves to the CNS by retrograde flow --> it will incubate for 3-12 weeks (can be up to a year) --> the animal will show signs of the disease and dies as early as 7 days from the first signs of symptoms
|
|
|
What characterizes the structure of rabies virus?
|
Contains 5 genes which each code for one protein: G (envelope), M (matrix), N (nucleoprotein encapsulating RNA), L (RNA-dependent RNA polymerase), P (phosphoroprotein) – the ribonucleoprotein core (RNP) consists of RNA, N, L, and P proteins
|
|
|
What do large amounts of accumulated nucleocapsids in neurons form?
|
Inclusion bodies, called Negri bodies
|
|
|
What are percutaneous absorption skin variables?
|
Stratum corneum thickness, cutaneous vasculature, area of absorptive surfaces
|
|
|
What are the topical retinoids?
|
Natural: All-trans retinol, All-trans retinoic acid, Alitretinoin; Synthetic: Tazarotene, Adapalene
|
|
|
What clinical effects are important regarding retinoids?
|
Enhance keratinocyte differentiation leading to decreased follicular occlusion; Comedolysis; Epidermal thickening; Dermal regeneration/collagen formation
|
|
|
What is an important pharmacological effect of retinoids?
|
Decreased sebum production
|
|
|
What is an important adverse effect of glucocorticoids?
|
Atrophy and striae formation
|
|
|
What are percutaneous absorption diseased skin variables?
|
Inflamed skin, (ulceration)
|
|
|
Which anatomic sites are the extremes of percutaneous absorptive capacity?
|
Highest: mucous membranes; Lowest: palmar/plantar skin
|
|
|
What concepts are related to the rate of diffusion of drugs across the skin?
|
Movement through the epidermis, concentration, relative tenacity of the drug binding to the vehicle with which it is given, thickness of the stratum corneum/barrier, surface area to which applied
|
|
|
What are percutaneous absorption vehicle variables?
|
Ointment > solution, occlusive vehicle improves hydration, irritancy (alteration in skin barrier)
|
|
|
What are percutaneous absorption drug variables?
|
Concentration in vehicle (not volume), lipophilicity, molecular size
|
|
|
What characterizes Doxycycline and Minocycline?
|
Broad-spectrum antibiotics; At 20mg bid: inhibition of inflammatory cytokines, inhibition of collagenases/gelatinases; At 50-100 mg/day or bid: bacteriostatic and same actions as with 20mg
|
|
|
What is Imiquimod used for?
|
Genital/perianal warts, actinic keratosis, superficial basal cell carcinoma
|
|
|
What is the mechanism of action of Imiquimod?
|
Induces the production of TNF-α, IFN-α, IFN-γ, and other cytokines
|
|
|
What is the use of 12% ammonium lactate?
|
Moisturizer: detachment or keratinocytes, normalization of keratinization; used in xerosis and ichthyosis vulgaris
|
|
|
What are the topical calcineurin inhibitors?
|
Tacrolimus, Pimecrolimus – used for atopic dermatitis in patients >2yo; must monitor for decades to assure safety
|
|
|
What is an important adverse effect of Minocycline?
|
Blue-black pigmentation of nails, skin, scars, sclera, teeth
|
|
|
What are important adverse effects of Doxycycline?
|
Phototoxic effect, brown discoloration of teeth in children
|
|
|
What are the systemic retinoids?
|
Isotretinoin: acne vulgaris; Acitretin (don’t take alcohol): psoriasis; Bexarotene: mycosis fungoides – all are category X
|
|
|
What characterizes Benzoyl peroxide?
|
Broad-spectrum bactericidal agents, effective against P. acnes (no resistance seen)
|
|
|
What adverse effects are seen with topical retinoids?
|
Teratogenicity, (erythema, irritation, sun sensitivity, desquamation)
|
|
|
What is the mechanism of action of Dapsone?
|
Inhibition of neutrophil myeloperoxidase, neutrophil adhesion, neutrophil chemotaxis
|
|
|
What adverse effects are associated with Thalidomide?
|
100% teratogenicity if fetus exposed days 21-36 of gestation --> phocomelia (deformity of arms, legs)
|
|
|
What are the uses of Thalidomide?
|
Erythema nodosum leprosum
|
|
|
What is the mechanism of action of Thalidomide?
|
Potent inhibition of TNF-α and IL-12
|
|
|
What adverse effects are associated with Dapsone?
|
Hemolytic anemia (check for G6PD deficiency), peripheral neuropathy
|
|
|
What is Dapsone used for?
|
Systemic: leprosy, dermatitis herpetiformis; Topical: acne vulgaris
|
|
|
How is the diagnosis of Botulism intoxication made?
|
Link to ingestion of home-canned or exotic food; Clinical appearance: blurred/double vision, dilated pupils, dry mouth, difficulty swallowing/speaking, weakness/dizziness, eventual effects on respiration/heart; Laboratory diagnosis: detection of organism or toxin, mouse neutralization test, PCR (for neurotoxin genes) – this is a reportable disease
|
|
|
What characterizes the epidemiology, treatment, and prevention of Botulism intoxication?
|
Majority of outbreaks traced to home-cooked foods; Treatment: early use emetics/gastric lavage, treat with trivalent anti-ABE toxin horse serum and supportive therapy (respirator/nourishment);
Prevention: destroy C. botulinum spores (pressure cooker), in acid pH spores do not germinate |
|
|
What are the effects of Botulism intoxication on the body?
|
Does not kill cells, but paralysis lasts for months (recovery requires formation of new nerve endings and connections at motor end-plates)
|
|
|
What is the mechanism of botulinum toxin?
|
Absorbed from intestine --> spreads by bloodstream --> binds to receptors on the presynaptic membrane of motor neurons of the PNS --> internalized by receptor-mediated endocytosis (RME) --> vesicle acidification causes release of LC into the motor neuron --> LC cleaves SNARE proteins (not the SNARE complex) resulting in inhibition of ACh release --> prevents muscle contraction --> flaccid paralysis
|
|
|
What characterizes normal neurotransmitter release?
|
Mediated by SNARE proteins: synaptobrevin interacts with syntaxin and SNAP 25 to cause fusion of the neurotransmitter vesicle with the pre-synaptic membrane (causing neurotransmitter release)
|
|
|
What characterizes botulinum toxin?
|
Most toxic substance in nature, heat-labile, AB-toxin; The B-subunit binds to ganglioside + protein receptors (synaptotagmin, found only on neural tissue), and forms the ion translocation channel; The A-subunit is a zinc metalloprotease (LC)
|
|
|
What are the virulence factors for Clostridium botulinum?
|
Neurotoxins (AB-toxins): there are 8 antigenic types (A-G, with A/B/E being most important); types A and B are chromosomal/plasmid encoded, whereas C and D are phage encoded
|
|
|
What characterizes Clostridium Botulinum?
|
Gram(+), long rods, spore-forming, obligate anaerobe
|
|
|
What characterizes Botulism intoxication?
|
Relates to ingestion of botulinum toxin (most deadly of food-borne illnesses); symptoms occur 12-36 hrs after ingestion, cranial nerves are affected first followed by respiratory paralysis;
25% mortality rate (if survive, frequently sustain neurological damage) |
|
|
What describes the transmission of Clostridium Botulinum?
|
Found in soil and water; spores on fruits/vegetables – majority of outbreaks traced to home-produced foods
|
|
|
By what route can wound botulism come about?
|
Administration of black tar heroin (“skin popping”)
|
|
|
What characterizes the disease caused by Clostridium tetani?
|
Tetanus/lockjaw: toxin-mediated disease (produced during bacterial growth, released upon lysis); has an 8-day incubation period;
Symptoms: stiffening of jaw muscles, severe/painful spasms of voluntary muscles, difficulty swallowing, tightening of muscles of abdomen/limbs, fever, sweating, high BP, irregular heart beat, muscle spasms can last 3-4 weeks; Complications: respiratory failure, spinal/long bone fractures; 10-78% mortality rate |
|
|
What characterizes Clostridium tetani?
|
Gram(+), spore-producing, drumstick appearance, obligate anaerobe;
Transmission: found in soil/animal feces, bacteria enter through wounds, no person-person spread; Diagnosis: isolate organism from wound, immunization history |
|
|
What is an additional use of botulinum toxin?
|
Bioterrorism agent
|
|
|
What complications are associated with Botox treatment?
|
Ptosis, development of immunity to toxin
|
|
|
What are therapeutic uses of botulinum toxin?
|
Treatment of muscle disorders, hyperhidrosis, myofascial pain, migraine HA, cosmetic treatment
|
|
|
What characterizes would botulism?
|
Caused by deep wound contamination with C. botulinum spores --> germinate and produce toxin;
Symptoms: paralysis begins at wound, then become generalized; Diagnosis: isolation of organism/ toxin from wound; Treatment: Ampicillin/Penicillin, surgical drainage, anti-ABE toxin serum |
|
|
What characterizes infant botulism?
|
Most common form of botulism in US; occurs in children < 9 months due to lack of development of normal flora; associated with ingestion of C. botulinum spores (mainly via honey); produce toxin which is transported to the blood stream
|
|
|
What is the treatment for infant botulism?
|
Supportive treatment (respiratory); BIG treatment: IV botulinum human immune globulin;
No antibiotics should be administered (!) – generally favorable outcome (mortality <2%) |
|
|
What are the symptoms of infant botulism?
|
FTT, “floppy baby” (flaccid paralysis); implicated as a cause of SIDS
|
|
|
What characterizes tetanus neonatorum?
|
Major cause of death in developing countries; Disease: due to infection of umbilical stump in infants born to nonimmune mothers – related to lack of aseptic technique during childbirth
|
|
|
What are the most common causes of tetanus?
|
Puncture wounds, IDU, laceration
|
|
|
What characterizes the prevention of tetanus?
|
Toxoid vaccine: formalin, alum-treated toxin; included in DTaP; requires booster every 10 years
|
|
|
What characterizes the treatment of tetanus?
|
Clean wound, tetanus immune globulin, Penicillin, toxoid immunization (inject at different site to prevent formation of immune complexes), supportive measures (barbiturates, tracheostomy)
|
|
|
What characterizes the epidemiology of tetanus?
|
Rare in US, due to widespread use of tetanus toxoid vaccine; Common world-wide – up to 40% carrier rate in humans (transient member of flora)
|
|
|
What are the differences between tetanus and botulinum toxin activity?
|
Tetanus toxin: acts on CNS to inhibit release of glycine/GABA --> spastic paralysis;
Botulinum toxin: acts on PNS to inhibit release of ACh --> flaccid paralysis |
|
|
What are the virulence factors of Clostridium tetani?
|
Tetanus toxin: heat-labile toxin, AB-toxin, similar to botulinum toxin – binds gangliosides on peripheral nerve endings at myoneural junctions, internalized by RME, transported to CNS; cleaves synaptobrevin in synaptic vesicles, preventing glycine/GABA release, causing muscle contraction --> spastic paralysis
|
|
|
Which people are given bacterial meningitis prophylaxis?
|
Those who have come in contact with a patient for 20hrs over 4-5 days; Medical personnel involved in intubation/resuscitation; Second case of H. flu in a day care within 60 days
|
|
|
What characterizes perinatal HSV infection?
|
Occurs at exposure to HSV secretions present in birth canal; risk of neonatal infection largely influenced by mother’s antibody status – maternal primary infection: 50% of neonates acquire; maternal secondary infection: 5% of neonates acquire
|
|
|
What etiologic agent is thought to be responsible for 85-90% of cases of aseptic meningitis?
|
Non-polio enterovirus
|
|
|
What sequelae are associated with encephalitis?
|
Mortality ranges from 0-80% depending on the agent; Morbidity is also dependent on the agent – many long-term sequelae are subtle: fine motor, discrete learning difficulties, partial hearing loss
|
|
|
What are viral etiologies of meningitis?
|
Enterovirus: by far the most common cause of meningoencephalitis and encephalitis;
HSV: common, treatable cause of encephalitis; Arbovirus |
|
|
What are etiologies of non-infectious encephalitis?
|
Collagen-vascular disorders, neoplastic, malignant HTN, toxin ingestion, metabolic disease
|
|
|
What sequelae are associated with bacterial meningitis?
|
Sensorineural hearing loss (20-30% with S. pneumo, 5-10% with Hib/Neisseria, CSF glucose<20 has worst prognosis); Seizures; Developmental delay/static encephalopathy; Hydrocephalus -
5-10% mortality overall in developed countries |
|
|
What characterizes neurocysticercosis?
|
Pork tapeworm cysts; cysts in brain cause seizures, behavioral disturbances, obstructive hydrocephalus, gait disturbances – leading cause of seizures in some parts of the world
|
|
|
What is the treatment for neurocysticercosis?
|
Avoid raw/undercooked beef/pork, treat those with adult GI tapeworm, sewage/hand hygiene;
Praziquantel, steroids, anticonvulsants |
|
|
What is ingested in order to get neurocysticercosis?
|
Pork tapeworm eggs (i.e. another person in shedding eggs fecally, and they are ingested orally)
|
|
|
How can perinatal HSV manifest itself?
|
On skin, eyes, mucous membranes: 7-14 days; CNS infection: 14-21 days, 15% mortality/54% sequelae if treated; Disseminated infection: 54% mortality, 38% sequelae if treated
|
|
|
What are the sequelae of TB meningitis?
|
Long term: blindness, deafness, intracranial calcifications, diabetes insipidus, obesity, paraplegia, mental retardation – 41% if diagnosed early, 92% if diagnosed after altered mental status;
poor prognosis if <4yo and with seizures |
|
|
What characterizes the CNS involvement with a TB infection?
|
95% meningitis, 2% tuberculomas, <1% abscess; 50% CNS involvement with miliary TB
|
|
|
What characterizes TB meningitis?
|
30% of children will have a positive PPD; Arises from lympho-hematogenous spread (2-6months after primary infection); Onset is gradual, over a period of 3 weeks; Most common in children less than 6yo (rare <4 months old, mean age 4yo)
|
|
|
What is the treatment for perinatal HSV?
|
Acyclovir – can cause crystallization in kidney, watch hydration
|
|
|
What are the four factors of the epidemiology of zoonoses?
|
Infective agent, host (vertebrate reservoir, human), transmission (vehicle, route), environment
|
|
|
What is the usual role of humans in zoonoses?
|
Usually a dead end in the chain of infection; person-person transmission can occur but is uncommon, examples include leishmaniasis, trypanosomiasis, viral encephalitides
|
|
|
What characterizes the epidemiology of Yellow fever?
|
Maintained in jungle: monkeys are primary reservoir hosts; forest species of mosquito acquire the virus and spread it to urban areas
|
|
|
What characterizes the epidemiology of plague?
|
200 different natural animal hosts; Primary reservoir hosts: rodents, transmission by fleas;
Secondary reservoir hosts (spread from sylvatic foci to urban foci): rats, mice, severe outbreaks |
|
|
What is the difference between transstadial and transovarial passage?
|
Transstadial: passage of microbe from one developmental stage of the host to its subsequent stage;
Transovarial: passage of microbe from maternal body to eggs within ovaries |
|
|
What are examples of arthropod reservoirs?
|
Rickettsia rickettsii: maintained for several years in tick, transstadial and transovarial passage;
Babesia microti: overwinters in tick vector, transmitted to rodents in spring |
|
|
What characterizes the primary and secondary reservoir hosts?
|
Primary: permanent reservoir of infection; Secondary: involved in natural cycle of infection, important role in spread to humans, not necessary for survival of infectious agent
|
|
|
What characterizes the Hantavirus Pulmonary Syndrome, 1993 outbreak?
|
98 cases, 52% fatality rate; transmitted by deer mouse feces (aerosolized)
|
|
|
What can be said about modern zoonoses?
|
Viruses have a greater potential due to their ability to adapt changing ecologic condition, development of previously uninhabited areas, and increased contact between man and animals
|
|
|
What defines zoonotic infections?
|
Infections that are transmitted in nature between vertebrate animals and humans – criteria: vertebrate reservoir exclusive of humans, transmission, infectious disease syndrome in humans
|
|
|
What are examples of diseases transmitted by animal contact?
|
Rabies, viral hemorrhagic fevers, Hantavirus pulmonary syndrome, Q fever, leptospirosis, tularemia, etc.
|
|
|
Which infections are considered occupational hazards?
|
Leptospirosis: rice field workers;
Anthrax: carpet weavers; Q fever: abattoir workers; Cutaneous larva migrans: plumbers |
|
|
What are examples of geoclimatic conditions that affect epidemiology of infectious agents?
|
Bacillus anthracis spores: alkaline soil containing calcium salts;
Vibrios of northern hemisphere: Winter in estuarine mud, significant numbers only at 20C; RMSF/Lyme disease: excluded from Alaska by cold |
|
|
What are examples of common vehicle-borne infections?
|
Food/milk: brucellosis, salmonellosis, tuberculosis; Water: leptospirosis
|
|
|
What vector-borne infections are transmitted by fleas and lice?
|
Fleas: plague, murine typhus; Lice: epidemic typhus, relapsing fever, trench fever
|
|
|
What are the most common vector-borne viruses (indirect)?
|
Arboviruses (stands for ARthropod-BOrne viruses): yellow fever, dengue fever, viral encephalitis
|
|
|
What characterizes manners of airborne transmission?
|
Droplet spread: direct spray onto conjunctiva, mucous membranes of mouth/nose, distance of up to 1 meter, droplet size > 5microns; Airborne transmission/droplet nuclei transmission: indirect, travel > 1 meter from source to host, droplet size < 5microns, animal or inanimate source of airborne droplet (e.g. Q fever, propagated by sheep/cattle, aerosolized particles)
|
|
|
In which diseases are humans the reservoir?
|
Plague and Yellow fever – promote rapid spread, responsible for fulminant outbreaks
|
|
|
What are various routes of transmission?
|
Contact, airborne, vector-borne, common vehicle (e.g. contaminated food)
|
|
|
What complications can be seen with Pasteurella multocida infection?
|
Osteomyelitis, tendonitis, bacteremia, can be life threatening
|
|
|
What characterizes ulceroglandular tularemia?
|
High fever, HA, prostration (loss of strength/exhaustion); Organisms gain entrance to host through lesion on skin, papule forms and ulcerates by 7th day, regional lymph nodes become painful and enlarge by 7th day (can suppurate)
|
|
|
What virulence factors are associated with Francisella tularensis?
|
Weak endotoxin; Able to survive and grow intracellularly in macrophages (major virulence factor)
|
|
|
What characterizes Francisella tularensis?
|
Small, Gram(-) rods, fastidious, facultative, require cysteine; cause Tularemia (rabbit fever)
|
|
|
What is the treatment for a Pasteurella multocida infection?
|
Penicillin
|
|
|
Which animal is the most common cause of bites in humans?
|
Dogs (followed by cats and other humans)
|
|
|
Where can Pasteurella multocida be found?
|
Oral flora of cats, dogs, pigs, horses, panthers, cougars – more prevalent in cat than dog
|
|
|
What zoonoses are associated with direct contact?
|
Pasteurella, Francisella, Bartonella
|
|
|
What is the hallmark of Pasteurella multocida infection?
|
Acute cellulitis: rapidly progressing, intense inflammation; can see regional lymphadenopathy and purulent discharge
|
|
|
What characterizes Pasteurella multocida?
|
Gram(-) coccobacillus; grows on blood agar with CO2; identified by biochemical tests; infection occurs after animal bites and scratches
|
|
|
What characterizes pneumonic tularemia?
|
Inhalation of organisms, highly fatal; can get cellular immunity with activated macrophages; live vaccine is available (not very good); diagnosis is made through fluorescent antibody of aspirate, PCR, serology, or culturing of aspirates (difficult and dangerous to grow)
|
|
|
How is cat scratch disease diagnosed?
|
History, PCR and serology (very high antibody titers!)
|
|
|
What are complications of cat scratch disease?
|
Encephalitis, oculoglandular syndrome, endocarditis
|
|
|
What characterizes cat scratch disease?
|
Fairly common; associated with kitten and cat scratches; self-limiting regional lymphadenopathy, often with fever; most common cause of chronic, benign adenopathy in young adults
|
|
|
What characterizes Bartonella?
|
Gram(-) rod; difficult to culture, very slow growing
|
|
|
What characterizes Bartonella infection?
|
Causes three disease: cat scratch disease (B. henselae), angiomatosis (B. henselae), trench fever (B. quintana)
|
|
|
What is the treatment for tularemia infection?
|
Ciprofloxacin, Streptomycin, or Gentamicin
|
|
|
What are 50% of cases of tularemia associated with?
|
Bite from deer fly or tick – similar to ulceroglandular tularemia, except that sometimes there is no local lesion
|
|
|
What characterizes typhoidal tularemia?
|
Drinking or eating contaminated food/water (eating undercooked rabbit, drinking water contaminated by beavers); causes a syndrome similar to typhoid fever
|
|
|
What characterizes oculoglandular tularemia?
|
Initial lesions near eye, with ulcer on eye-lid
|
|
|
What is the most common arthropod-borne disease in the US?
|
Lyme disease; caused by the spirochete Borrelia burgdorferi: multiplies in ticks and animals, it has a small linear chromosome with linear and circular plasmids
|
|
|
What is the treatment for Lyme disease?
|
Doxycycline early on; Ceftriaxone in later stages – Vaccine: recombinant OspA (OspA is expressed in ticks, but only transiently expressed in humans);
Prevention: Doxycycline 72hrs after tick bite |
|
|
How is the diagnosis of Lyme disease made?
|
Clinical findings: erythema migrans, cardiac abnormalities, arthritis, neurologic manifestations;
Laboratory tests: ELISA using whole cell antigens, Western blot using whole cell antigens |
|
|
What are the virulence factors of Lyme disease?
|
No endotoxin; Lipoproteins induce inflammation; VlsE lipoprotein: undergoes antigenic variation due to gene rearrangement; Differential gene expression allows it to grow in two different hosts (tick and animals); Motility
|
|
|
What are the three stages of Lyme disease?
|
Stage 1: bull’s eye rash (erythema migrans, 1-30 days after tick bite, seen in 60-80% of cases), fever, malaise, lymphadenopathy; Stage 2: neurological manifestations (cranial nerve palsies, arthritis, cardiac abnormalities, 2-8 weeks after bite); Stage 3: chronic disease (arthritis, often oligoarthritis, auto-immune disease), neurological (encephalopathy, peripherally neuropathy, chronic fatigue)
|
|
|
What describes the Ixodes tick life cycle?
|
Larvae (gets blood meal) --> nymph (responsible for most transmission)--> adult --> eggs
|
|
|
What characterizes the transmission of Lyme disease?
|
East: Ixodes scapularis, deer, mice; West: Ixodes pacificus, muskrat, deer, mice – infects host if tick bites at least for 48hrs
|
|
|
What is the treatment for cat scratch disease?
|
Azithromycin, Erythromycin
|
|
|
What characterizes trench fever?
|
Caused by B. quintana; transmitted by lice and animal contact (seen in crowded/homeless war-time condition); can see chills, fever, rash, splenomegaly, bacteremia, endocarditis; can be mild or severe with relapses; organisms replicate on surface of animal cells; treatment: Erythromycin
|
|
|
What characterizes angiomatosis?
|
Occurs in immunocompromised patients (e.g. AIDS); can see swelling of blood vessels, due to endothelial proliferation after engulfment; can also see fever, bacteremia, liver/spleen infection – transmitted by contact with cats; treatment: Erythromycin
|
|
|
What drug is derived from Pacific Yew Bark?
|
Paclitaxel (for breast/ovarian cancer) – 10-DAB from yew shrub needles can be synthesized into Paclitaxel and Docetaxel
|
|
|
What characterizes biotin?
|
It is the active group for a variety of carboxylation reactions (e.g. pyruvate carboxylase, acetyl carboxylase, propionyl-CoA carboxylase)
|
|
|
What characterizes pantothenic acid?
|
It is a portion of the coenzyme A (CoA) as well as a portion of fatty acid synthase; required for the metabolism of proteins, fats, carbohydrates – deficiency of pantothenic acid resembles the deficiency state of other B vitamins
|
|
|
What characterizes vitamin B12 (cobalamin)?
|
Vitamin B12 is the extrinsic factor important in pernicious anemia (whereas its endogenous counterpart is intrinsic factor, both are needed for B12 absorption); the methylated form of B12 converts homocysteine to methionine; the 5-deoxy adenosyl derivative causes methyl malonyl CoA to be converted to succinyl CoA
|
|
|
What characterizes vitamin B3 (niacin)?
|
It can be synthesized from tryptophan (though it is not produced in adequate amounts); it is a precursor of NAD and NADP; Nicotinic acid (i.e. niacin) causes flushing with taken orally and lowers lipids in the blood
|
|
|
What does a cobalamin deficiency lead to?
|
Degradation of the myelin sheath (due to defects in fatty acid metabolism) --> neurological s/s;
Hematological defects (require both B12 and intrinsic factor) --> pernicious anemia |
|
|
Who should be concerned about getting enough iron?
|
Pre-adolescent children, and pre-menopausal women – supplemental iron intake in adult men or post-menopausal women can be a cardiovascular risk
|
|
|
What characterizes copper?
|
Important element in metalloenzymes; Deficiency lead to hypercholesterolemia, demineralization of bones, leukopenia, etc.; Excess seen in Wilson’s disease
|
|
|
What characterizes zinc?
|
Crucial substance in enzymes and gene factors, necessary for growth/maintenance of the body
|
|
|
What are unsafe herbs and their reactions?
|
****one exam question from this list!
Chaparral (cancer): hepatotoxicity; Comfrey (wounds): hepatotoxicity (!); Ephedra (weight loss): high BP, arrhythmias; Lobelia (asthma): CNS over-stimulation; Pennyroyal (abortion): CNS toxicity; Yohimbe (ED): CNS over-stimulation; Germander (tonic): hepatotoxicity; Stephandra (tonic): bradycardia |
|
|
What characterizes Ephedra?
|
Chinese stimulant “warming” herb, contains ephedrine/pseudoephedrine; long-used as a bronchodilator for asthma in China; Popular for weight loss, weight lifting; Removed from market by FDA due to the dangers of overdose: elevated BP, tachycardia, arrhythmias, stroke
|
|
|
What does an iron deficiency lead to?
|
Iron deficiency anemia (most prevalent dietary deficiency), causes a microcytic/hypochromic anemia
|
|
|
Which herbs/supplements are not thought to have a benefit?
|
Echinacea; Shark cartilage; Ephedra/Mah Huang (safety concerns!); Vitamin E; Dong quai;
Beta-carotene |
|
|
Which herbs/supplements are thought to have benefit?
|
Green tea catechins; Flavonoid-rich juices: grape, pomegranate, acai; Omega 3 fatty acids;
St. John’s Wort (for mild depression); A healthy diet rich in fresh fruits and vegetables |
|
|
What cannot be done under DSHEA 1994?
|
No claim can be made for a dietary supplement to treat, cure, or mitigate a disease; however, it can make claims that if affects or maintains the structure or function of the body
|
|
|
What drug is derived from Goat’s Rue?
|
Biguanides, a derivative of which includes Metformin (for DM)
|
|
|
What is homeopathy?
|
Diluted herbal/medicine essence (e.g. diluted poison ivy essence)
|
|
|
What can star anise seeds be used for?
|
Contain Shikimic acid, which is a vital precursor in the manufacture of Oseltamivir (for influenza treatment) – can also get Shikimic acid from Ginkgo biloba
|
|
|
What is pharmacognosy?
|
The scientific study of natural product medicines
|
|
|
Which herb may improve symptoms of the common cold and acute bronchitis?
|
Pelargonium sidoides root, from a South African geranium
|
|
|
What characterizes Panax Ginseng?
|
Root is used as an adaptagen (tonic herb) for decreased stamina and fatigue; Ginsenosides are the active component; Side effects: elevated BP, nervousness, insomnia, estrogenic effects, hypoglycemia; Caution: HTN, CV disease, avoid in pregnancy or with steroids, possible “ginseng abuse syndrome”; Status: may be useful for improved sense of well-being or energy
|
|
|
What characterizes Kava?
|
Used for anxiety (GABA receptor modifier) and stress reduction; contains methysticin and kavain; Side effects: decreased reflexes and judgement, kava dermatitis (flaking discolored skin), idiosyncratic liver toxicity; Caution: psychological dependence (avoid with alcohol, benzodiazepines, levodopa); Status: effective with some abuse and liver injury potential
|
|
|
What characterizes St. John’s Wort?
|
Contain hypericin/hyperforin which may inhibit neurotransmitter reuptake and activate GABA receptors to treat mild depression, it is not an MAOI at usual dose; SJW induces CYP3A4 (!);
Side effects: photosensitivity, nausea, dry mouth, restlessness, mania; Caution: SSRI/MAOI (serotonin syndrome), pregnancy, alcohol, P450 drug interactions |
|
|
What characterizes Milk Thistle Seed?
|
Used for hepatotoxic ingestions, and as a cytoprotectant/antioxidant/anti-inflammatory;
Side effects: loose stools; Status: liver protector used IV in Amanita mushroom poisoning in Europe, may shield liver from toxins but so far no evidence for chronic viral hepatitis or alcoholic cirrhosis |
|
|
What characterizes Saw Palmetto?
|
Widely used for nocturia due to BPH; contains fatty acids thought to be anti-inflammatory and a weak 5-alpha reductase inhibitor; Very slow-acting but effective in the majority of studies;
Side effects: n/d/HA; No effect on PSA and doesn’t shrink prostate |
|
|
What characterizes Ginkgo biloba?
|
Ginkgo extract contains flavonol glycosides and terpene lactones; should be taken at least 8 weeks before assessing efficacy; Ginkolic acid must be removed from Ginkgo lead before use to prevent seizures; It is used as a vasodilator for aged arteries and for mental alertness (though it probably doesn’t improve memory); Terpene lactone inhibits platelet activating factors and may increase bleeding risk if used with other anti-coagulants
|
|
|
What is the difference between intrinsic and extrinsic adverse effects?
|
Extrinsic: failure of good manufacturing practices leads to adverse effects;
Intrinsic: Type A (predictable toxicity, overdose, interaction with drugs), Type B (idiosyncratic reactions, like allergy/anaphylaxis) |
|
|
What characterizes Echinacea?
|
Native American herb and garden flower, root extract and leaves of three related species;
Immune stimulant or “booster”; causes tongue to tingle if potent; No clinical benefit for cold prevention, mixed cold treatment results; appears safe but not effective |
|
|
What are infusions, decoctions, macerations, tinctures, and poultices?
|
Infusion: near-boiling water poured on herb; Decoction: simmer herb and strain;
Maceration: steeping herb in room temp. water; Tincture: steeling herb in ethyl alcohol and water; Poultice: herb paste application |
|
|
What characterizes Phytoestrogens?
|
They are plant sterols that bind to estrogen receptors (SERMs, weak effect); used to control menopausal symptoms as an alternative to HRT; Soy/legumes contain isoflavones (genistein, daidzein), flax seed contains lignans, clover contains coumestans; May have favorable impact on lipids and cardiac risk
|
|
|
What negative effect is associated with Green Tea?
|
It interferes with the ability of Bortezomib to kill tumor cells (e.g. in the treatment of glioblastoma and multiple myeloma)
|
|
|
What is Green Tea extract FDA approved for?
|
Genital warts (HPV)
|
|
|
What characterizes Green Tea (Camellia sinensis)?
|
Contains catechin polyphenols (Epigallocatechin gallate – EGCG), which is a powerful antioxidant; Has an anticancer effect (EGCG inhibits growth of leukemia cells, reduces risk of esophageal cancer, and inhibits aryl hydrocarbon receptors), may help prevent Alzheimer’s disease, as well as cause enhanced weight loss, a reduction of tooth decay, contain anti-inflammatory properties, help against HPV, and reduce CV risk
|
|
|
What characterizes Resveratrol (grape extractions)?
|
It is a phytoalexin (found in Japanese knotweed roots and grape skin/red wines); Binds to and stimulates the Sir2 sirtuin enzyme, mimicking the effect of caloric restriction on longevity;
May reduce heat and cancer risk; research is mixed for mammalian longevity |
|
|
What describes the effectiveness of various antioxidants?
|
Vitamin E: doesn’t seem to help prevent CAD or cancer, and may increase all cause mortality;
Beta-carotene: failed to prevent cancer in smokers and increased mortality; Vitamin C: doesn’t reliably prevent colds but does help prevent post-fracture bone pain – regular doses of anti-oxidants may actually increase mortality (except for vitamin C or selenium) |
|
|
What substances are thought to function as antioxidants?
|
Green tea, vitamins E/C, grape seen extract, pycnogenol, turmeric, milk thistle, plant pigments (carotenoids, lycopene, flavonoids), statins (from red yeast rice)
|
|
|
What characterizes Garlic?
|
Used to improve lipid profiles, as an antibacterial, anti-thrombotic, anti-hypertensive, anti-inflammatory, and anti-cancer food; Active ingredient is Allicin; Only fresh Allicin is an effective anti-infective; Garlic increases NO synthesis, thus reducing the likelihood of blood clotting (should only be used as an adjunct for BP/cholesterol treatment); Excessive amounts cause gastritis or increase bleeding risk with Coumadin; Mixed results for decrease in cholesterol/BP
|
|
|
What characterizes the functions vitamin A?
|
Forms a dimer (beta carotene); essential for normal vision, prevention of night blindness, epithelial cell maintenance/differentiation, bone growth, body development, immune functioning
|
|
|
What characterizes vitamin K?
|
Consists of three different quinones (phylloquinone, menaquinone, menadione – vit. K1-3, resp.); Needed in the synthesis of four proteins involved in blood clotting: F2 (prothrombin), F7, F9 (plasma thromboplastin), F10 (Stuart factor); vitamin K causes gamma-decarboxylation of specific glutamic acid residues producing gamma carboxyglutamic acid, which is involved in chelation of ions (e.g. calcium, which allow prothrombin to become active) – vitamin K is also involved in bone healing/remodeling
|
|
|
What characterizes vitamins E?
|
A group of 8 substances called tocopherols and tocotrienols; they are fat-soluble antioxidants (Alpha D tocopherol is the most potent, but the others can prevent nitration damage);
they prevent red cell hemolysis and nerve damage |
|
|
What does a vitamin D deficiency result in and what about an excess?
|
Deficiency: rickets; Excess: hypercalcemia --> muscle weakness, bone pain, anorexia, HTN, arrhythmias
|
|
|
What characterizes the formation of vitamin D?
|
Formed from ergocalciferol added to cow’s milk --> irradiated by UV light to vitamin D3 --> hydroxylated to 25-hydroxy-vitamin D by the liver --> hydroxylated to the active 1,25-dihydroxy-vitamin D by the kidney
|
|
|
What vitamin A derivatives are used clinically?
|
All-trans retinoic acid: acne, keratosis, pilaris, acute promyelocytic leukemia; Acitretin: psoriasis; Isotretinoin: acne; Adapalene: acne
|
|
|
What do excessive amounts of vitamin A lead to?
|
Toxicity to skin, spleen, kidneys, liver; bone thickening, pain
|
|
|
What are the vitamins?
|
Fat soluble: D, E, A, K (a deficiency state can occur when the patient has problems with the absorption of fats); Water soluble: B1-3, 6, 12, C, pantothenic acid, folic acid
|
|
|
Which vitamins can act as antioxidants and how?
|
C, E – they form a redox cycle with glutathione causing the oxidized form (R-SS) to become reduced (R-SH); reduced glutathione is a major cellular antioxidant
|
|
|
Which vitamin is toxic in high doses?
|
A
|
|
|
What does a thiamine deficiency cause?
|
Beriberi: mental confusion, ataxia (milder symptoms incl. depression, peripheral neuropathy, irritability, fatigue); seen in severe alcoholism
|
|
|
What does a folate deficiency lead to?
|
Decreased DNA synthesis --> cellular arrest (in S phase), produces a megaloblastic anemia;
since folate is used for the conversion of homocysteine to methionine, hyperhomocysteinemia can occur; Inadequate intake during pregnancy can lead to neural tube defects |
|
|
What characterizes folic acid?
|
Free folic acid is reduced to tetrahydrofolate, which carries a methyl group on its N5 nitrogen; this methyl group is used in the biosynthesis of choline, serine, purines, and pyrimidines
|
|
|
What does a deficiency of pyridoxines lead to?
|
Depression, irritability, nervousness, microcytic anemia with high serum iron (since pyridoxal phosphate is needed to synthesize a precursor for heme biosynthesis), glucose intolerance, increased homocysteine (pyridoxines play a role in the conversion of homocysteine to cysteine)
|
|
|
What characterizes vitamin B6-complex (pyridoxines)?
|
Converted into pyridoxal phosphate, which is needed for the catabolism/interconversion of amino acids; also needed for the synthesis of neurotransmitters (5-HT/NE/sphingolipids for myelin prod.)
|
|
|
What does a deficiency of riboflavin lead to?
|
Angular cheilitis, glossitis, scaly dermatitis; seen in chronic alcoholism
|
|
|
What characterizes vitamin B2 (riboflavin)?
|
Structural component of the coenzymes FAD and FMN, which are necessary for energy production and respiration
|
|
|
What does a deficiency of vitamin K result in?
|
Increased prothrombin time (can happen in newborns and in adults using excessive anticoagulants), and possibly osteoporosis
|
|
|
What characterizes vitamin B1 (thiamine)?
|
Converted into active compounds that function in energy production via glycolysis and Krebs cycle
|
|
|
Why is toxicity with water-soluble vitamins rarely found?
|
They are excreted by the body
|
|
|
What does a niacin deficiency lead to?
|
Deficient DNA repair, since large amounts of NAD are required for the enzyme PARP, which is involved in nick-repair – give in time-release form for continuous supplies;
Deficiency leads to glossitis and pellagra (with severe deficiency): dermatitis, diarrhea, dementia |
|
|
What can magnesium deficiency lead to?
|
Tetany
|
|
|
What characterizes calcium?
|
Most abundant of body minerals; essential part of bone formation, but also required for many enzymatic reactions incl. blood coagulation and muscle function; if calcium levels are insufficient, PTH causes bone dissolution which can lead to osteoporosis if calcium intake over time is low
|
|
|
What does an ascorbic acid deficiency lead to?
|
Mild: pinpoint hemorrhages under the skin (caused by increased capillary fragility);
Severe: scurvy (causes decreased wound healing, osteoporosis, hemorrhaging, anemia) |
|
|
What characterizes vitamin C (ascorbic acid)?
|
Antioxidant; functions in hydroxylation of lysine/proline (collagen synthesis, maintenance of connective tissue, wound healing); concentrated in the adrenal gland where it may be linked to steroid hydroxylation reactions or in stabilizing DA/NE/Epi; aids in the absorption of iron by reducing it to the ferrous state in the stomach – vitamin C overdose is not significant, except for causing kidney stones in certain susceptible individuals
|
|
|
What is the treatment for Lyme disease?
|
Tetracycline (e.g. Doxycycline)
|
|
|
What can be done once rabies symptoms appear in a human?
|
Essentially nothing (almost 100% mortality rate)
|
|
|
How long should a potentially rabid animal be observed?
|
10 days; if they survive past 10 days, they probably do not have rabies
|
|
|
What characterizes Congo-Crimean hemorrhagic fever?
|
*****learning point
Tick-born viral hemorrhagic disease, spread from domestic animals (e.g. sheep) and wild animals; causes a capillary leak syndrome – treat with Ribavirin |
|
|
What is the treatment for rickettsial illness?
|
Doxycycline
|
|
|
What characterizes Typhus (African tick typhus)?
|
*****learning point
A group of infectious diseases caused by rickettsiae, transmitted by arthropods (e.g. ticks) – will see a patient with an eschar, fever, and relative bradycardia |
|
|
What effect on the heart a lots of intracellular pathogens associated with?
|
Bradycardia ( –will see a patient with a fever, but no tachycardia)
|
|
|
What are the important families of spirochetes?
|
Borrelia (Lyme disease), Treponema (Syphilis), Leptospira (Leptospirosis)
|
|
|
Which spirochete can be seen on peripheral smear?
|
*****learning point
Only Borrelia |
|
|
Which spirochete is associated with relapsing/remitting fever?
|
Borrelia – due to antigenic variation
|
|
|
Which disease is associated with a “break-bone fever”?
|
Dengue fever – can also see a petechial rash
|
|
|
What characterizes pneumonic plague?
|
*****learning point
A rapidly progressive and often fatal form of plague which can be transmitted person-person |
|
|
In which disease do patients present with swollen lymph glands, called buboes?
|
Bubonic plague – caused by Yersinia pestis
|
|
|
What is the treatment for Dengue?
|
Supportive treatment only
|
|
|
What is the natural reservoir for Dengue?
|
Monkeys – virus is transmitted by the Aedes mosquito
|
|
|
What are the two types of Dengue?
|
Dengue fever: hurts but doesn’t kill; Dengue hemorrhagic fever: hurts and kills
|
|
|
What is the most widely distributes mosquito-borne infection?
|
*****learning point
Dengue |
|
|
What should be done after someone has been bitten by a rabid animal?
|
*****learning point
Prompt post-exposure prophylaxis: give Ig and vaccinate |
|
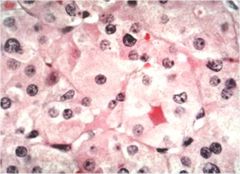
How is the diagnosis of rabies infection made?
|
In animal: biopsy brain; In human: biopsy neck skin – look for Negri bodies
|
|
|
What signs/symptoms are associated with rabies?
|
Either agitation or depression
|
|
|
What characterizes septicemic plague?
|
Sepsis, DIC, high mortality rate
|
|
|
What does the disease presentation of tularemia compare to?
|
That of plague – ulcer at site of inoculation and swollen lymph nodes
|
|
|
What disease is associated with open wounds and rabbits?
|
Tularemia – caused by Francisella tularemia
|
|
|
What is the treatment for plague?
|
*****learning point
Aminoglycosides |
|
|
What organisms are involved in the transmission of Yersinia pestis?
|
Rodents and fleas
|
|
|
What are the potential side effects of herbal medicines?
|
Allergic reactions, interaction with prescription drugs
|
|
|
What are the adverse effects of particulates?
|
Ultrafine particles (< 0.1 microns) are the most hazardous --> may cause pulmonary/systemic inflammation, arrhythmias due to autonomic effects, and increased blood viscosity (!)
|
|
|
What are the adverse effects of SO2?
|
In water releases acidic substances (H+, bisulfite, sulfite) --> upper/lower airways irritation
|
|
|
What are the adverse effects of Ozone, NO, NO2?
|
Forms hydrogen peroxide (and lipid aldehydes) on respiratory mucosa --> inflammation, bronchoconstriction (exacerbates asthma) – as little as 0.8 ppm during exercise can cause cough, chest discomfort, and lung inflammation; NO and NO2 release acidic substances in water causing upper respiratory irritation but are less reactive than ozone
|
|
|
What are the 6 major pollutants EPA monitors regularly?
|
Ozone, Nitrogen oxides, Sulfure oxides, Particulates, Carbon monoxide, Lead (!)
|
|
|
What are the major sources of ambient air pollution?
|
Fossil fuels, Photochemical reactions that make ozone, Power plants (create particulates, SO2/acid rain which irritates airways), Waste incinerators/smelters/industry (metals, mercury, acid aerosols)
|
|
|
What group of volatile organic compounds is used widely in dry cleaning?
|
Aliphatic hydrocarbons (e.g. chloroform, carbon tetrachloride, methylene chloride, perchloroethylene)
Perchloroethylene (used in dry cleaning): potential carcinogen, CNS depression/confusion, dermatitis |
|
|
Where does absorbed lead in children end up in?
|
Bone and teeth (80%) with a half-life of 30 years in bone – Lead found in blood only indicates recent exposure to Lead as it is cleared rapidly from the blood
|
|
|
What is the most important route of exposure to Lead?
|
Occupational setting: inhalation (!) ***
Consumer setting: ingestion with greater absorption in infants/children and greater absorption with Ca, Fe, Zn deficiencies (Lead poisoning can begin in utero) |
|
|
What toxic chemicals are used in the rubber/plastic industry?
|
Occupational: Vinyl chloride --> angiosarcoma of liver - 1,3 butadiene --> leukemia
Public: Phthlate --> testicular injury in rate – Bisphenol-A --> estrogenic proliferative effects |
|
|
How do polycyclic aromatic hydrocarbons (3 or more fused benzene rings) cause cancer?
|
Their metabolites bind to DNA (e.g. scrotal cancer in chimney sweeps in 1700s)
Prototype carcinogen is benzo[a]pyrene --> present in cigarettes, can cause bladder and lung cancers |
|
|
What aromatic hydrocarbon is considered a carcinogen?
|
Benzene: its CYP450 metabolites, benzoquinone and muconaldehyde, are toxic to the bone marrow --> aplastic anemia, acute leukemia
|
|
|
What is caused by inhalation of vapors of petroleum products (gasoline, kerosene, turpentine)?
|
Dizziness, incoordination, CNS depression
|
|
|
What are the major effects of air pollutants?
|
Major effect on lungs – children, asthmatics, and individuals with chronic lung/heart disease are esp. prone
|
|
|
What are the adverse effects of Radon and Asbestos?
|
Radon: lung cancer in as many as 10,000 per year in the US
Asbestos: lung cancer, mesothelioma Fiberglass (replacement for asbestos): irritant but not a carcinogen |
|
|
What has been the trend in the levels of indoor air pollutants?
|
Increasing levels due to more insulation and less ventilation of homes aimed at saving energy costs
Examples: CO, NO2, wood smoke (includes polycyclic aromatic hydrocarbons), formaldehyde, benzene, polycyclic aromatic hydrocarbons, radon (in basements), asbestos, fiberglass, bioaerosols (Legionella, allergens, sick building syndrome due to poor ventilation) |
|
|
What is the correlation between serum Lead levels in children and IQ levels?
|
Inverse correlation (even at chronic levels <10 ug/dL can cause mental retardation)
|
|
|
What characterizes dioxins?
|
At low doses present in food, soil, water; at high doses may cause leukemia, lymphoma, sarcomas
Used in Agent Orange in the Vietnam war |
|
|
What are organophosphates?
|
Irreversible cholinesterase inhibitors: cause abnormal transmission in CNS/PNS, absorbed via skin/GI/lungs, 40% of farm workers have inhibition of RBC or plasma cholinesterase activity
Carbamates: reversible cholinesterase inhibitors with similar effects (Carbaryl: potentially teratogenic) |
|
|
What are organochlorines?
|
Insecticides that have low acute toxicity for humans; absorbed via GI, skin, lungs; examples include
DDT (metabolite DDE): persists in environment, accumulates in food chain/fat, found in human milk Chlordane: possible lymphoma in farm workers; Lindane |
|
|
What characterizes pesticide toxicity to humans?
|
Toxic to humans at high concentrations, herbicides have the lowest toxicity among pesticides; rodenticides possibly have the highest toxicity (death)
|
|
|
What is an adverse effect of various metals besides Lead?
|
Cobalt and Tungsten Carbide: Interstitial lung fibrosis (hard metal disease);
Cadmium: (acutely) lung edema and irritation, (chronically) kidney PCT damage; Chromium: Cr3+ is carcinogenic by DNA damage and free-radicals; Nickel: (mainly inhaled) carcinogenic by DNA damage, (if topical) contact dermatitis |
|
|
What is the correlation between serum Lead levels in females and puberty?
|
As little as 3 ug/dL can delay puberty in females
|
|
|
What is an early and characteristic finding in Lead poisoning?
|
Hypochromic anemia: Lead inhibits enzymes that catalyze incorporation of Iron into heme, leading to reduced heme biosynthesis
|
|
|
What is a blood test for (chronic) Lead poisoning?
|
Elevated serum Zinc protoporphyrin, free RBC protoporphyrin (!)
Serum Lead is not measure as it only indicates acute exposure to Lead |
|
|
What are the various biochemical effects of Lead?
|
Bone: competes with Ca --> stores in bone;
CNS: competes with Ca --> interferes with nerve transmission and brain development; Hypochromic anemia: inhibits Iron incorporation into heme --? reduced heme synthesis Inhibit membrane-associated enzymes --> decrease RBC survival, kidney damage, HTN Impaired production of 1,25-diOH Vitamin D |
|
|
What natural toxins are produced by the nature?
|
Fungi: mycotoxins (may contaminate food), e.g. Aflatoxin in peanuts --> carcinogen, liver cancer;
Plants: phytotoxins (may contaminate food); Animals: dinoflagellate toxins ciguatoxin (paresis) and saxitoxin (paralysis) ingested by eating fish/snails/mollusks, e.g. paralytic shellfish poisoning (saxitoxin: neurotoxin, paralysis) |
|
|
How many drugs does the average geriatric patient take?
|
3.2 drugs (women 3.5, men 2.8); 40% take more than three drugs;10% take more than five drugs’; 65% of total national usage of cardiovascular and diuretic drugs are consumed by the elderly
|
|
|
When % of hospital admissions in the geriatric population is due to adverse drug effects or nonadherence?
|
28%
|
|
|
What describes the geriatric pharmacokinetics of absorption?
|
Decreased gastric acid production (increased pH, usually due to comorbid illness); reduced splanchnic (intestinal) blood flow; decreased GI motility
|
|
|
What describes the geriatric pharmacokinetics of Distribution and Metabolism?
|
Lean body mass (vs. adipose tissue) declines w/ age as does plasma volume; Lipophilic drugs (benzodiazepines, lidocaine) are distributed more extensively, thereby prolonging half-life. Greater effect in F than M as F develop relatively less lean body mass w/ age; Water soluble drugs (acetaminophen) will not distribute as widely, therefore higher serum concs could occur; Decreased serum albumin (more likely in ill, frail, poorly nourished --> Protein-bound drugs (phenytoin, warfarin, thyroxine, NSAID) may be more available as free drugs, hence, toxic effects)
|
|
|
How is Hepatic metabolism changed in Geriatric Pharmacokinetics?
|
Phase 1 metabolism (oxidation, reduction, hydrolization): decreases w/ age; metabolites are as effective or more effective than the parent drug & are more water soluble; Phase 2 metabolism (conjugation to inactive metabolites) does not decrease w/ age (1st pass effect may yield more drug to the circulation as hepatic metabolism is diminished)
|
|
|
What drugs have increased bioavailability in the elderly?
|
Propranolol*, Labetalol, Levodopa*, nifedipine, Omeprazole, odansetron
|
|
|
What describes the geriatric pharmacokinetics of Elimination?
|
Renal metabolism declines with age and is worsened with disease, i.e., HTN (nephrosclerosis), CHF (decreased renal blood flow), diabetes (nephropathy); Renal function (creatinine clearance) over age 65 is about 1/2 that at age 20; Creatinine may not worsen as there is decreased lean body mass
|
|
|
What important drugs must be used with caution in patients with renal impairment?
|
Digoxin, aminoglycosides, cimetidine, lithium, penicillin
|
|
|
What changes in the CNS are associated with normal aging?
|
Decrease in muscarinic receptors; Increase in catechol O methyl transferase (COMT); Increase in Monamine oxidase ( MAO)
|
|
|
What changes in the Cardiovascular system are associated with normal aging?
|
Decreasing sensitivity of beta adrenergic receptors
|
|
|
What changes in the Respiratory system are associated with normal aging?
|
Decreased awareness of hypoxia and hypercapnia
|
|
|
What changes in the GI system are associated with normal aging?
|
Decrease in Vitamin D absorption, Slower transit times usually due to comorbid illness, medication and lifestyle.
|
|
|
What changes in the eye are associated with normal aging?
|
Ciliary atrophy, rigidity of lens and iris
|
|
|
What changes in the skin are associated with normal aging?
|
Decreased ability to sweat
|
|
|
What changes in the urologic system are associated with normal aging?
|
Decrease in contractility of bladder; Increase in size of prostate
|
|
|
What changes in the GFR are associated with normal aging?
|
Decrease in GFR --> Decrease in ability to excrete metabolites: Sodium and potassium
|
|
|
What are predictors of therapeutic failure in the geriatric population?
|
Multiple comorbid illness (i.e CHF, renal failure, Alzheimer’s); Poor functional ability (i.e. activities of daily living)
|
|
|
What therapeutic considerations must be taken into account with geriatric patients?
|
Diminished memory (complicated schedules, drug names, forgetting a dose or doubling); Costs; Psychiatric conditions commonly depression (subtle Sx, may mimic dementia, Overdose or refusal to take meds); impaired vision; Swallowing function (decreasing esophageal motility, worse GE reflux, achalasia, strictures, impaired salivation; i.e. Alendronate)
|
|
|
What are some common pitfalls in geriatric therapy?
|
OA (PT & acetaminophen best 1st choice, not NSAIDS); CA chemotherapy (Best predictor of success is severity of comorbid illnesses & functional ability- not age); Anxiety (Often coexists w/ depression, best 1st choice is SSRI, not benzodiazepine)
|
|
|
What is “Beers list”?
|
Some medications are statistically associated w/ ADE, Usually medications with anticholinergic effects, toxic metabolites, or long half lives and potential for significant side effects
|
|
|
What medications are inappropriate medications in older people* (independent of diagnosis)?
|
Propoxyphene; Indomethacin; Phenylbutazone; Pentazocine; Trimethobenzamide; Methocarbamol, carisoprodol, oxybutynin, chlorzoxazone, metaxalone, cyclobenzaprine; Flurazepam; Amitriptyline; Doxepin; Meprobamate; hlordiazepoxide, diazepam (long-half life benzo); Disopyramide; Dipyridamole; Methyldopa; Reserpine
|
|
|
What herbal medications are inappropriate medications in older people?
|
Dicyclomine, hyoscyamine, propantheline, belladonna alkaloids; Chlorpheniramine, diphenhydramine, hydroxyzine, cyproheptadine, promethazine, tripelennamine; dexchlorpheniramine; Ergot mesyloids; Ferrous sulfate supplements >325 mg/day
|
|
|
If a geriatric pt is experiencing new symptoms, what should always be considered?
|
That it is possibly being drug-related
|
|
|
What is the treatment for S. aureus bacteremia/endocarditis/pneumonia?
|
Nafcillin (if sensitive); if MRSA use Vancomycin – if sensitivities are unknown (i.e. initially) use Nafcillin plus Vancomycin
|
|
|
What is the treatment for streptococcus pyogenes pharyngitis?
|
Penicillin – if allergic use Azithromycin
|
|
|
What is the treatment for streptococcus pyogenes TSS/necrotizing fasciitis?
|
Clindamycin plus Penicillin G
|
|
|
What is a good empiric therapy for endocarditis?
|
Vancomycin plus Gentamicin
|
|
|
What is the treatment for enterococcus endocarditis/bacteremia?
|
IV Ampicillin plus Gentamicin (low dose) – if allergic to Ampicillin, give Vancomycin
|
|
|
What is the treatment for streptococcal infective endocarditis?
|
High dose Penicillin G – if allergic, give Vancomycin
|
|
|
What is the treatment for an IV line infection with coagulase-negative staphylococci?
|
Vancomycin
|
|
|
What are causes of bacterial meningitis and what is their treatment?
|
S. pneumo/N. meningitidis: Vancomycin plus Ceftriaxone; L. monocytogenes: Ampicillin
|
|
|
What are causes of community-acquired pneumonia and what is the treatment?
|
S. pneumo: Ceftriaxone; Mycoplasma pneumoniae/Legionella: Azithromycin
|
|
|
What is the treatment for rickettsial infections (and ehrlichia)?
|
Doxycycline – give right away when rickettsial infection is suspected (don’t wait for cultures)
|
|
|
What is the treatment for intraabdominal/pelvic infections?
|
Gentamicin (for Gram(-) rods) + Metronidazole (for anaerobes) + Ampicillin (for enterococcus) – alternatively, Metronidazole can be replaced by Ampicillin/Sulbactam or Clindamycin
|
|
|
What is the treatment for inpatient cellulitis?
|
IV Vancomycin (scale down therapy if possible) – In the case of a diabetic lower extremity or with a suspected mixed infection: Vancomycin plus Ceftriaxone plus Metronidazole
|
|
|
What is the treatment for outpatient cellulitis?
|
If known MSSA: Cephalexin; If unknown or MRSA: TMP/SMX, Doxycycline, Clindamycin (if sensitive); If GAS: Dicloxacillin, Clindamycin
|
|
|
What is the treatment for otitis media and sinusitis (S. pneumo/H. flu)?
|
1st line: Amoxicillin, TMP/SMX, Azithromycin; 2nd line: Amoxicillin/Clavulanate, Cefuroxime
|
|
|
What is the treatment for Chlamydia trachomatis?
|
Azithromycin (or Doxycycline)
|
|
|
What is the treatment for Neisseria gonorrhea?
|
Ceftriaxone
|
|
|
What is the treatment for syphilis (T. pallidum)?
|
Benzathine Penicillin IM (or aqueous Penicillin G, if neurosyphilis)
|
|
|
What is the treatment for UTI in a young, otherwise healthy woman?
|
Cystitis (E. coli): Trimethoprim/Sulfa (TMP/SMX) – if pregnant use Amoxicillin;
Pyelonephritis (Gram(-) rods, enterococcus): Ampicillin (enterococcus) + Gentamicin (E. coli) |
|
|
Who should not take Fluoroquinolones (e.g. Ciprofloxacin)?
|
Children under 16, and in pregnancy
|
|
|
What is the treatment for pseudomonas aeruginosa infection?
|
Use 2 drugs: Aminoglycoside + Ceftazidime (or Piperacillin/Tazobactam, Cefepime or Imipenem) – if patient can’t take β-lactams use Aminoglycoside plus Ciprofloxacin
|
|
|
What adverse effects are associated with Vancomycin?
|
"Red man” syndrome”: immediate reaction, histamine release; Phlebitis, rash, bone marrow suppression, ototoxicity, nephrotoxicity
|
|
|
What adverse effects are associated with Synercid (Quinupristin/Dalfopristin)?
|
↑LFTs, myalgias, arthralgias
|
|
|
What are Synercid, Linezolid, and Daptomycin used for?
|
VRE and MRSA
|
|
|
What adverse effects are associated with Doxycycline?
|
Photosensitivity rash; ↑LFTs
|
|
|
What adverse effects are associated with Trimethoprim/Sulfamethoxazole (TMP/SMX)?
|
Rash, bone marrow suppression (esp. ↓WBC), ↑creatinine
|
|
|
What adverse effects are associated with Metronidazole?
|
Antabuse reaction, peripheral neuropathy, ataxia
|
|
|
What adverse effects are associated with Macrolides (Erythro-/Azithro-/Clarithromycin)?
|
May lead to QT prolongation; n/v/d, liver enzyme elevations – Clindamycin: diarrhea (including C. difficile), rash
|
|
|
What adverse effects are associated with β-lactams (Penicillins, Cephalosporins)?
|
Anaphylaxis; Rashes, n/v/d, interstitial nephritis; With high-dose, prolonged therapy: bone marrow suppression – Imipenem (less so Meropenem) may cause seizures, so avoid in patients with CNS pathology, seizure disorders, and renal failure
|
|
|
What adverse effects are associated with Quinolones (Ciprofloxacin, Levofloxacin)?
|
Nausea, CNS effects (hallucinations, confusion, seizures); May lead to QT prolongation;
Tendon rupture, arthropathy; Photosensitivity (esp. newer quinolones), hyper-/hypoglycemia |
|
|
What adverse effects are associated with Aminoglycosides?
|
Renal toxicity; Ototoxicity (vest/aud); rarely, neuromuscular blockade
|
|
|
What adverse effects are associated with Linezolid?
|
Many drug interactions; Low WBC/platelets; Lactic acidosis; Retinopathy
|
|
|
What antimicrobials are considered safe in pregnancy (category A/B)?
|
β-lactams (Penicillin/Cephalosporins), Azithromycin/Erythromycin
|
|
|
What antimicrobials should be avoided in pregnancy?
|
Doxycycline/Tetracycline; Quinolones
|
|
|
What adverse effects are associated with Daptomycin?
|
↑creatinine kinase/LFTs, myopathy
|
|
|
What are the toxic effects of Acetaldehyde and how are Asian effected?
|
Nausea, flushing, tachycardia, hyperventilation, 50% of Asians have low ALDH activity and do not metabolize acetaldehyde well (low alcohol tolerance)
|
|
|
Increased incidence of cancer of oral cavity, larynx, esophagus and liver are all associated with what toxicity?
|
Increased incidence of cancer of oral cavity, larynx, esophagus and liver (hepatocellular carcinoma); May be related to acetaldehyde
|
|
|
How does a baby with Fetal Alcohol Syndrome present at birth?
|
Microcephaly, growth retardation, facial abnormalities, Reduction in mental functions, 0.1 to 0.5% of U.S. births
|
|
|
How does chronic alcoholism cause malnutrition?
|
Empty calories and Vitamin B1 deficiency (Peripheral neuropathies, Wernicke-Korsakoff syndrome)
|
|
|
How does Chronic Alcoholism affect the Liver, Upper GI tract, Pancreas, CV system, & CNS?
|
Liver (Fatty change, Alcoholic hepatitis, Cirrhosis, Hepatocellular carcinoma);
Upper GI Tract (Bleeding gastric ulcer, Esophageal varices); Pancreas (Acute and chronic pancreatitis); CV system (Dilated congestive cardiomyopathy, Hypertension); CNS (Cerebral atrophy, Cerebellar degeneration) |
|
|
What are the acute & chronic effects of alcohol abuse?
|
Acute (reversible: CNS depression, Gastritis and gastric ulcer, Fatty liver [hepatic steatosis]);
Chronic alcoholism (Morbidity, Shortened life span) |
|
|
What causes the accumulation of fat in the liver in of an alcoholic?
|
Depletion of NAD which is required for fatty acid oxidation
|
|
|
How is ethanol absorbed?
|
Unaltered in stomach and small intestine, Evenly distributed to all tissues and fluids of body, 10% excreted intact in urine, sweat, and breath, Basis of breath test
|
|
|
What effect does alcohol have on the CYP enzyme system?
|
At high blood alcohol levels, the CYP system also converts ethanol to acetaldehyde, it is induced 5 to 10-fold in chronic alcoholics & may accelerate metabolism of other drug; Unless there is a high blood alcohol level, which competes for CYP, delays drug metabolism, and potentiates the depressant effects of narcotics, sedatives, etc.
|
|
|
What describes the metabolism of alcohol?
|
Most blood alcohol is converted to acetaldehyde in the liver, catalyzed by alcohol dehydrogenase (ADH), this rxn also converts NAD to NADH, Acetaldehyde is converted to acetic acid by acetaldehyde dehydrogenase (ALDH), Acetic acid is then used for energy source in the mitochondrial respiratory chain
|
|
|
What was the significance of the Women’s Health Initiative study, 2002 about HRT?
|
Increased risk of breast cancer and thromboembolism; No reduction in cardiovascular disease; Reduction in number of fractures
|
|
|
What is the antidote for Acetaminophen toxicity in the first 12 hours?
|
N-acetylcysteine, which restores GSH, tapering off the excess levels of NAPQ
|
|
|
How does Acetaminophen use lead to hepatocellular injury and necrosis?
|
Small proportion metabolized by CYP to NAPQI, a highly reactive & toxic metabolite, which is normally conjugated & detoxified with glutathione (GSH); In larger doses, GSH is depleted, & NAPQI accumulates & binds covalently to hepatic proteins causing injury
|
|
|
What drug causes almost 50% of cases of acute liver failure, has a mortality rate 30%, & may require liver transplant for survival?
|
Acetaminophen
|
|
|
What is the major adverse effect of Acetaminophen and Aspirin?
|
Acetaminophen: Hepatic necrosis (at large doses) (!); Aspirin overdose: respiratory alkalosis, metabolic acidosis; Aspiring chronic toxicity (3 grams/day): tinnitus, erosive gastritis, bleeding, analgesic nephropathy
|
|
|
What are the ADR in Anabolic Steroids?
|
Males: Testicular atrophy, gynecomastia, acne;
Females: Facial hair, menstrual changes; also: Premature MI, hepatic cholestasis, psychiatric problems |
|
|
What are the adverse effects from use of Oral Contraceptives?
|
Thromboembolism (Overall, a 3-fold increased risk, Increased synthesis of coagulation factors (seems to be induced by OC));
Cardiovascular disease (Smokers have increased risk of MI, Nonsmokers have increased risk of MI after age 35): Cancers (Reduced incidence of endometrial & ovarian CA (not an ADR!), Small increased risk of breast CA in 1st 5 yrs); Increased risk of Hepatic Adenoma (benign) |
|
|
What are the beneficial effects caused by moderate consumption of alcohol and red wine?
|
Increased HDL levels, decreased platelet aggregation, Red wine contains resveratrol, may promote longevity
|
|
|
What is the consequence of using Estrogen only Hormonal Replacement Therapy?
|
Increased risk of endometrial cancer, so used only post-hysterectomy
|
|
|
How many hospitalized pts are affected by Adverse Drug Reactions & how many of them die?
|
Affect 10% of hospitalized patients, Of whom 10% die (implies that 1% of all hospitalized pt will die of an ADR)
|
|
|
What are the symptoms of toxicity with Acetaminophen?
|
Pretty non-specific, Nausea, vomiting, diarrhea, sometimes shock, Jaundice in a few days
|
|
|
What drug can cause Analgesic Nephropathy?
|
Tubulointerstitial nephritis with renal papillary necrosis caused by long-term use of combinations of aspirin and phenacetin (acetaminophen) in large quantities
|
|
|
What are the morphological changes associated with chronic aspirin toxicity?
|
Acute erosive gastritis;
Gastric ulcer; Petechiae in skin and viscera; Bleeding tendency |
|
|
How does Acute salicylate overdose present?
|
Early respiratory alkalosis (Hyperventilation from stimulation of respiratory center);
Metabolic acidosis later (Uncoupling of oxidative phosphorylation, Accumulation of pyruvate and lactate); In larger doses there are CNS effects (range from nausea to coma) |
|
|
What can cause Inadvertent poisoning when combined w/ Aspirin?
|
Excess use of ointment with oil of wintergreen (methyl salicylate) (otherwise overdose is usually suicidal)
|
|
|
What are examples of particulate radiation?
|
Alpha: 2 neutrons + 2 protons (e.g. Radon gas) --> strongly ioninzing, low penetration;
Beta: electrons --> less ionizing, more penetrating than alpha |
|
|
How does oxygen level of tissue become a factor in the damage caused by radiation?
|
Hyperbaric oxygen increases cell injury by gamma and x-rays as ionizing radiation produces oxygen-radicals (may be counteracted by antioxidants)
|
|
|
What is the most significant subcellular target of radiation?
|
DNA: rapidly dividing cells are more radiosensitive (e.g. hematopoietic, GI, squamous epithelia, endothelial, lymphocytes), whereas quiescent cells are not as sensitive (bone, cartilage, muscle, peripheral nerve)
|
|
|
What are the most significant factor in the biological effect of radiation?
|
Dose rate: single dose of 40 is more damaging than 10 doses of 4 (less time for cellular repair)
Whole body radiation: more damaging than regional doses with shielding Cell cycle and cell type: G2 and mitotic phases are most sensitive |
|
|
How do gamma and alpha rays differ in their radiation energy transferred?
|
Alpha: produce heavy damage in a restricted area by interacting with many molecules over a short distance
Gamma: penetrate deeply, dissipating energy more gradually over a longer distance, while producing less damage per unit of tissue |
|
|
What units are commonly used in radiation?
|
Gray: measures energy absorbed by target tissue per unit mass, expressed as ergs/gram ; Sievert: unit of dose that measures biologic effect ; Different types of radiation differ in the degree of tissue damage they produce, even at the same physical dose (Gray); The equivalent dose (Sievert) corresponds to the absorbed dose (Gray) multiplied by relative biologic effectiveness of the radiation; For X-rays, 1 Sievert=1 Gray
|
|
|
What is radiation?
|
Electromagnetic energy which at short wavelengths (high frequency) acts as particles and at long wavelengths (low frequency) acts as waves; radiation exposure is 80% from natural sources (cosmic, UV, natural radioisotopes such as Radon gas), 20% from manufactured sources (medical imaging, radio waves, microwaves)
|
|
|
What is ionizing radiation?
|
Short wavelength (high frequency), high-energy radiation that may be unsafe and ionize/eject electrons from molecules; examples include X-rays, gamma rays, cosmic radiation
|
|
|
What is non-ionizing radiation?
|
Long wavelength (low frequency), low-energy radiation that is safe and produces only vibration/rotation of atoms; examples include electric, radio, microwaves, IR, UV (!)
|
|
|
What is the most important delayed effects of radiation?
|
Blood vessels are among tissues most vulnerable to delayed radiation injury
Inflammation of blood vessels --> endothelial cell necrosis --> luminal narrowing by fibrosis -->occlusion --> ischemic changes to organs supplied (fibrosis, atrophy); this may occur at doses used for cancer treatment |
|
|
What are the delayed or chronic effects of radiation to the following organs?
|
Skin: epidermal atrophy, dermal fibrosis, dilation of SC vessels --> poor healing, ulceration, SCC, BCC;
Heart: fibrosis of heart/pericardium --> MI, CHF, constrictive pericarditis due to fibrosis; Lung (acute: 1-6 months post-treatment): edema, inflammation, dyspnea; Lung (delayed radiation pneumonitis: months to years post-treatment): dyspnea, chronic cough, decreased lung function, fibrosis, increased incidence of lung cancer; Kidneys: tumors of kidney; fibrosis of vessels/glomeruli --> HTN, atrophy; Bladder: tumors of bladder; epithelial cell necrosis, submucosal fibrosis --> ulcers, bleeding from bladder; GI (acute): exfoliation of mucosa --> loss fluids/electrolytes, inflammation; GI (chronic): small vessel injury --> ischemia (ischemia of bowel shown on slide 48); Breast: diagnostic doses during adolescence increase breast cancer incidence in 15-20 years; Breast: radiation therapy for breast cancer --> dense fibrosis, pleomorphism of epithelial cells, radiation-induced sarcoma; Ovary and Testis: spermatogonia extremely sensitive, acute degeneration of ovarian follicles; Eyes: cataracts (lens is sensitive) (!) *****; Brain: focal necrosis, demyelination of white matter; Spinal cord: transverse myelitis with paraplegia below the level of injury (!) ****** |
|
|
In what manner is radiation used for therapy?
|
Doses given in fractions while shielding adjacent normal structures
Used to shrink tumor, relieve pain, or alleviate compression of adjacent structures Side effects include: radiation sickness, decreased PMNs/platelets, sterility, 2’ cancers, fibrosis (delayed radiation injury), fatigue/n/v |
|
|
What is caused by acute whole-body radiation?
|
Radiation sickness (acute radiation syndrome), potentially lethal, causes four clinical syndromes depending on severity of radiation: subclinical (prodormal: nausea, vomiting) --> hematopoietic --> GI --> CNS
|
|
|
Which cancers are most commonly seen as a result of radiation injury?
|
Population at large: skin cancer, osteogenic sarcoma, leukemia, lung cancer
Children: breast, thyroid, GI, urinary tract |
|
|
What is the latent period for cancer development following radiation?
|
10-20 years: due to accumulation of mutations from genomic instability caused by radiation
|
|
|
What is the most important acute effect of radiation?
|
DNA damage (cross-links, breaks) directly from radiation or indirectly via oxygen-radicals
|
|
|
What are the effects of radiation on cells?
|
High doses: necrosis;
Intermediate doses: killing of proliferating cells; Low doses: no histopathologic effects, but subcellular damage occurs to DNA, some undergo apoptosis --> surviving cells may have delayed effects (mutations, malignancy, delayed organ dysfunction by vascular damage or atrophy) |
|
|
What characterizes damage due to UV radiation?
|
Acute effects (low dose): erythema, pigmentation (UVA oxidizes melanin --> transient darkening);
Acute effects (high dose): erythema, edema, acute inflammation from histamine release; Chronic effects: tanning (UVA and UVB --> increased # of melanocytes, transfer of melanin to keratinocytes); UVB tanning is more protective for subsequent UV exposure than UVA tanning |
|
|
What can repeated UV exposure lead to? *******
|
Degeneration of collagen and elastin (irreversible), solar elastosis, irregular pigmentation --> premature sking aging; Triggers UV response pathways, which may be protective via inducing DNA repair, cell cycle arrest, and apoptosis (!)
|
|
|
What are the causes of injury from the physical environment?
|
Mechanical force, heat/cold, electric injuries, high altitude
|
|
|
What factors determine the character of a gunshot wounds?
|
Type of gun (rifle, handgun, shotgun: the longer the barrel, the more bullet energy), ammunition (caliber), distance from body, location of injury, trajectory of bullet (right angle or oblique), gyroscopic stability of bullet (tumbling bullets cause more injury)
|
|
|
What characterizes incisions?
|
Incision: sharp cutting object (knife) causing tear in skin, with clean margins and no bridging strands, margins readily approximated by suture, leaves little scarring
|
|
|
What characterizes lacerations?
|
Laceration: blunt force causing irregular tear in skin due to over-stretching, can involve deep tissue organs without involving the skin (e.g. spleen laceration), can be stellate or linear, shows bridiging blood vessels, and fibrous tissue strands, hemorrhagic margins (look at slide 9)
|
|
|
Which age group is especially prone to large hematomas from contusions?
|
Elderly due to small vessel fragility
|
|
|
What characterizes contusions?
|
Contusion: blunt force causing bruising without skin disruption, bruise caused by small vessel damage and interstitial bleeding; usually seen right away if superficial, but may not be evident if deep (swelling and tenderness may be only signs)
|
|
|
What are the various types of injuries from mechanical forces?
|
Abrasion, contusion, laceration, incision, gunshot wounds
|
|
|
In what age category is trauma a major cause of death?
|
Up to age 44 – includes accidental injuries, homicides, suicides
|
|
|
What are the biggest disparities in adult mortality rate of blacks compared to whites and Hispanics?
|
Death from HIV and homicide are almost 10-fold higher
|
|
|
What characterizes abrasions?
|
Abrasion: scrape, superficial epidermis torn off by friction or force, regenerates w/o scarring
|
|
|
What characterizes exit injuries from gunshot wounds?
|
Larger and more irregular than entrance wounds
|
|
|
What complications occur with large burns?
|
Greater than 50%: potentially fatal
Greater than 20%: release of osmotically active materials from dying cells --> fluid shift to interstitium --> increased interstitial osmotic pressure --> hypovolemic shock, generalized edema, pulmonary edema |
|
|
What is the hypermetabolic state that is caused by large burns?
|
Excess heat loss and increased need for nutrition --> increase in resting BMR --> breakdown of tissues to meet caloric needs if insufficient nutrition --> protein breakdown --> starvation (to preven, keep room temperate elevated, provide aggressive nutritional support)
|
|
|
What is the leading cause of death from in burn patients?
|
Organi failure from sepsis due to secondary infection of burns (main organisms: Pseudomonas aeruginosa, Candida, antibiotic-resistant S. aureus) (!)
|
|
|
What are the possible complications of thermal burn?
|
% body surface involved, airway/lung injury, 2ndary infection (leading cause of death), hypermetabolic state
|
|
|
What are the grading criteria for thermal burns?
|
Partial thickness (pink or mottled, blistered or painful);
1st: epidermis only; 2nd: superficial dermis (dermal appendages intact, can regenerate); Full thickness (white or charred, dry and anesthetic); 3rd degree: full thickness skin; 4th degree: subcutaneous |
|
|
What factors determine the clinical significance of thermal burns?
|
Depth, % body surface involved, internal injuries from inhaling hot/toxic fumes
|
|
|
How are close gunshot wounds different from long-range gunshot wounds?
|
Within 1 foot: shows fouling (gray discoloration of skin from burned powder), stippling (unburned powder);
1 to 3 feet: shows stippling without fouling; Greater than 3 feet: no fouling or stippling |
|
|
What characterizes internal injuries from gunshot wounds?
|
Low velocity bullets: burrowing, through and through (i.e. bullet goes through, no other damage in its path);
High velocity bullets: laceration of viscera (i.e. bullet causes much more damage in viscera in addition to going through) |
|
|
What characterizes entrance injuries from gunshot wounds?
|
Entrance is slightly smaller than bullet, may have narrow rim of abrasion, if shot at angle may be asymmetric, at close range may have lacerations of skin due to entering gas elevating the skin
|
|
|
What characterizes heat cramps?
|
Loss of electrolytes from sweating --> cramping of voluntary muscles with exercise – body has normal core temperate
|
|
|
What factors determine the type of injury caused by electrical injury?
|
Current intensity;
Electrical resistance of tissues (greater resistance --> greater heat): Dry skin (high resistance) --> surface burn; Wet skin (low resistance) --> ventricular fibrillation or respiratory paralysis current intensity |
|
|
What are hypothermias direct and indirect effects on body?
|
Direct: physical disruption of organelles, high salt concentration due to crystallization of ICF/ECF water;
Indirect (circulatory changes): if slowly developing, will get vasoconstriction, increased permeability, edema, “trench foot”; if rapidly developing, will get more rapid vasoconstriction, ischemia of toes and feet (frost bite), peripheral nerve damage, edema evident only after rewarming |
|
|
What occurs with severe hypothermia (90°F)?
|
Loss of consciousness, bradycardia, atrial fibrillation (arrhythmia is cause of death)
|
|
|
What temperature in heat stroke is associated with >50% mortality?
|
≥ 106°F
|
|
|
What characterizes heat stroke?
|
High ambient temp and humidity --> stopped sweating --> core body temperature rises --> marked peripheral vasodilation, peripheral pooling of blood, hypovolemia --> muscle/myocardium necrosis, arrhythmia, DIC
|
|
|
What characterizes heat exhaustion?
|
Hypovolemia from water loss --> sudden weakness and collapse --> quick recovery of equilibrium
|
|
|
What fluids should be used to reverse the hypovolemic shock caused from large burns?
|
Osmotically active fluids: albumin, plasma (not normal saline)
|
|
|
What is caused by hyperthermia?
|
Heat cramps --> heat exhaustion (most common) --> heat stroke (most dangerous)
|
|
|
How do lungs/airways get injured in thermal burns?
|
Direct effect of heat, toxic components in smoke (water soluble (chlorine, sulfur oxides) --> upper respiratory, bronchial injury;
lipid soluble (nitrous oxides) --> lower respiratory, lung injury) |
|
|
What is an example of a high intensity current that can cause electrical injury?
|
Lightning – injury is characterized by lightning marks (linear arborizing burns)
|
|
|
What is the treatment for decompression disease?
|
Repressurize --> depressurize gradually
|
|
|
What symptoms develop within hours of rapid decompression?
|
Respiratory difficulty, Substernal pain due to gas emboli in lungs (“the chokes”)
Headache, visual problems, disorientation, vertigo due to inner ear involvment (“the staggers”) Periarticular gas bubbles, joint pain (“the bends”) Delayed aseptic necrosis of femoral/humeral head due to embolic occlusion of blood supply, may cause degenerative joint disease eventually |
|
|
What characterizes decompression (caisson) disease?
|
O2 and N2 dissolved in blood at high pressure come out of solution as tiny gas bubbles if decompression is too rapid (O2 redissolves quickly) --> N2 and Helium bubbles can form emboli
|
|
|
What are injuries related to changes in atmospheric pressure?
|
High-altitude illness: low O2 tension --> confusion, increased capillary permeability, pulmonary edema
Blast injury (air blast, immersion/water blast): violent increase in pressure --> thorax collapse, abdominal compression, visceral rupture, alveoli damage, decompression wave with sudden expansion of abdomen and thoax may rupture intestines/lungs Decompression (caisson) disease (aka barotraumas): occurs in deep sea waters, underwater workers with rapid ascent |
|
|
How is Rickettsia rickettsii transmitted?
|
Eastern US: dog tick (Dermacentor variabilis); Western US: wood tick (Dermacentor andersoni) – most cases occur outside of the Rocky Mountains
|
|
|
How is Rickettsia prowazekii transmitted (Epidemic typhus fever)?
|
By the human louse Pediculus humanus; lives on human blood; Louse bites infected human --> louse becomes infected --> louse bites another human and defecates (feces contain Rickettsia) --> human scratches and becomes infected – once the human dies, the lice leave the host
|
|
|
What is the treatment for RMSF?
|
Initiate antibiotic treatment as soon as possible (within 5 days of illness); Tick removal with forceps
|
|
|
How is the diagnosis of RMSF made?
|
Clinical diagnosis is most important: history of tick bite, spring/early summer; can also do an agglutination tests of the patient’s serum, as well as fluorescent antibody testing of biopsied tissue
|
|
|
What characterizes the symptomatology of RMSF?
|
Incubation period: 2-6 days; Signs/symptoms: intense HA, rash (palms), fever, irritable, eventual coma and death; The severe vasculitis it causes starts on the wrists/ankles --> palms/soles --> up the extremities (some pts have no rash); can also see thrombocytopenia and hyponatremia
|
|
|
When does transmission of Rickettsia rickettsii occur once the tick latches on?
|
Within 4-6 hours as the tick becomes engorged
|
|
|
How does Rickettsia rickettsii from the tick to the human?
|
Either via transovarial transmission (to offspring and then into the human) or via an intermediate infected animal (to another tick and then into the human)
|
|
|
What are the obligate intracellular parasites and how are they transmitted?
|
Rickettsia: Dermacentor ticks; Coxiella: aerosol-inhalation; Ehrlichia: Amblyomma ticks; Anaplasma: Ixodes, Dermacentor ticks; (and Chlamydia: STD)
|
|
|
What are the two groups of Rickettsia?
|
Spotted fever group: always produces rash, affect endothelial and smooth muscle cells, includes Rickettsia rickettsii (causative agent of Rocky Mountain Spotted Fever – RMSF);
Typhus fever group: sometimes produce rash, affect endothelial cells, includes Rickettsia prowazekii (Epidemic typhus fever) and Rickettsia typhi/prowazekii (Endemic typhus fever) |
|
|
What characterizes Rickettsia?
|
Gram(-), non-motile, slow-growing; Parasitize animal for ATP
|
|
|
What characterizes Q fever?
|
Caused by Coxiella burnetii; Transmission occurs via aerosolized particles (cells are resistant to desiccation) which can come from contaminated milk, hides, dried feces, amniotic fluid, and placental tissues (!); High numbers are associated with birthing of infected cattle, sheep, goats
|
|
|
What characterizes Human granulocytic ehrlichiosis (HGE)?
|
Caused by Anaplasma phagocytophilia; Transmitted by the Deer tick (Ixodes scapularis) and Dog tick (Dermacentor variabilis); Diagnosis: inclusion in granulocytes, IFA, serology (at later stage); Treatment: Doxycycline
|
|
|
What characterizes Human monocytic ehrlichiosis (HME)?
|
Caused by Ehrlichia chaffeensis; Transmitted by the Lone star tick (Amblyomma), seen in the South (esp. with golfer); Causes leukopenia and thrombocytopenia; Diagnosis: IFA, serology (at a later stage); Treatment: Doxycycline
|
|
|
What signs/symptoms are associated with Ehrlichia and Anaplasma?
|
Fever, HA, ↑liver enzymes, myalgia, leukopenia, thrombocytopenia
|
|
|
How does Coxiella burnetii multiply in the human host?
|
After being endocytosed, fusion with the lysosome activates Coxiella and allows it to multiply inside of the phagolysosome
|
|
|
How is Q fever diagnosed, and what is the treatment?
|
Diagnosis: serology, PCR; Treatment: Tetracycline
|
|
|
What are the signs/symptoms of Q fever?
|
Fever, intense HA, malaise, chills, pneumonitis; can lead to endocarditis and death
|
|
|
How is the diagnosis of Epidemic typhus fever made?
|
Rash on trunk, stupor; Seen in Africa and S. America – Prevention: sanitary conditions; Treatment: Tetracycline
|
|
|
What is Endemic typhus fever associated with?
|
Rat flea, flying squirrels
|
|
|
Why is aspirin contraindicated with hemorrhagic fever?
|
It prevents clotting, leading to increased bleeding
|
|
|
What is the treatment for Dengue?
|
Hydration, avoid aspirin
|
|
|
What are preventative measures for Dengue?
|
Wear DEET; wear clothes that cover arms, legs, feet; close unscreened doors/windows; No good vaccine available
|
|
|
How is the laboratory diagnosis of Dengue made?
|
Leukopenia, thrombocytopenia, elevated serum transaminases; ELISA, RT-PCR (during acute phase), test for viral antigen in blood, test for virus in growth culture; measure an increase in antibody titer over acute vs. convalescent serum
|
|
|
Which Dengue infection is most severe?
|
Reinfection with a second serotype: due to the production of some non-protective antibodies during the initial infection causing greater uptake of viruses into monocytes/macrophages during the second infection (this gives the virus greater access to macrophages, where they reproduce) –
also occurs in concurrent infection with two serotypes |
|
|
What describes the signs/symptoms of Dengue?
|
Incubation period: 3-14 days; May be asymptomatic; s/s: fever, chills, severe HA, myalgias, bone and joints of very painful (breakbone fever), rash with severe itching, leukopenia, thrombocytopenia, elevated serum transaminases; Severe manifestations: Dengue hemorrhagic fever, Dengue shock syndrome
|
|
|
What characterizes Dengue?
|
Replicates in the cytoplasm of monocytes/macrophages; Transmitted by the mosquito Aedes aegypti (feeds on humans and primates); can also undergo transovarial transmission; It is the most prevalent arboviral disease; there are 4 serotypes (protection against one serotype does not mean protection against the others)
|
|
|
What three manifestations are seen with arboviral infections?
|
Hemorrhagic fever (small vessels petechiae and intestinal hemorrhage); CNS involvement (meningitis, meningoencephalitis); Liver damage
|
|
|
What describes the replications of Flaviviruses?
|
Viral RNA is translated into a large polyprotein --> the first protein is cleaved and acts as a protease --> the protease cleaves the polyprotein to generate capsid proteins and polymerase --> polymerase makes the (-)RNA strand which serves as the template for viral nucleic acids
|
|
|
What characterizes Flaviviruses?
|
(+) ssRNA, enveloped, no polyA tails; Includes Dengue, Yellow Fever virus, West Nile virus, Tick-borne Encephalitis virus, St. Louis Encephalitis virus
|
|
|
What describes the symptomatology of WNV?
|
Incubation period: 3-14 days; ~80% are asymptomatic; ~20% get “West Nile Fever”; <1% will develop CNS disease (10% of which is fatal); West Nile Fever consists of fever, chills, HA, fatigue (can be severe), n/v, rash (usually not itchy, lasting a few days, mainly on chest/back/ abdomen/arms) – persistent fatigue is common and may continue for months
|
|
|
What is the treatment for WNV?
|
None available (α-IFN being tested)
|
|
|
How is the diagnosis of WNV made?
|
Suspect in meningitis, encephalitis, flaccid paralysis from summer through fall (or December in the South); should also consider other arboviral diseases (St. Louis/La Crosse); History of recent travel; Test serum of CSF for WNV IgM/IgG
|
|
|
What characterizes WNV-associated flaccid paralysis?
|
Affects relatively healthy young people; may not have fever/HA before paralysis; Clinical hallmarks: onset early in infection, weakness can often be in only one limb, absence of numbness, pain sometimes present
|
|
|
What age group is WNV more so seen in?
|
Elderly
|
|
|
What characterizes WNV encephalitis?
|
More likely than meningitis patients to have memory problems, dysarthria, dysphagia, focal motor abnormalities (incl. cranial nerve palsies); severity ranges from mild confusion to coma/death
|
|
|
What characterizes WNV meningitis?
|
More likely than encephalitis patients to have myalgias, arthralgias, n/v, rash , back pain; can see WBC in CSF; HA may be quite severe; Most people improve though persistent symptoms may include fatigue, weakness, and memory/concentration problems
|
|
|
What characterizes West Nile virus?
|
Has only recently made its way to the US; Transmission occurs through the mosquito;
birds (crows and blue jays) are considered amplifying hosts (i.e. they allow for massive viral reproduction), after which mosquitoes become more likely to infect humans; Most cases occur late summer and in the fall |
|
|
Besides mosquito bites, what other modes of transmission exist for WNV?
|
Blood transfusions (now tested for viral nucleic acid), organ transplantation, intrauterine infection, breast milk (probable)
|
|
|
How does transmission of Dengue and West Nile virus (WNV) differ?
|
The mosquitoes associated with Dengue bite mostly early during the day, whereas those associated with WNV bite more commonly during dusk and in the evening
|
|
|
What characterizes Yellow Fever?
|
Also known as black vomit fever; Transmitted by Aedes species (incl. A. aegypti); very common disease; Undergoes two cycles: monkey --> human (jungle fever), human --> human (most common); can also see transovarial transmission
|
|
|
What is the symptomatology associated with La Crosse encephalitis?
|
Fever, HA, n/v, lethargy; severe disease incl. seizures, coma, paralysis, disorientation, increase in cranial nerve pressure, meningitis; in some pts, long-term behavior and cognitive deficits persist
|
|
|
What describes the epidemiology and transmission of La Crosse encephalitis?
|
WV is among states where it is most common (50% of US cases); seen in heavily wooded areas where Aedes triseriatus and rodents live together; Chipmunks and squirrels serve as amplifying vectors; can be transmitted transovarially
|
|
|
What characterizes La Crosse encephalitis (a Bunyavirus) and its replication cycle?
|
(-) ssRNA virus, 3 segments, enveloped; one segment codes for the replicase; Enter cell by RME --> virus ribonucleoprotein escapes into the cytoplasm, then into the nucleus --> (-) strand of RNA transcribed into (+) strands --> viral encoded RNA polymerase generates (-) strand viral genomes --> assembled viruses escape cell by budding, with plasma membrane lipids
|
|
|
What are prevention measures regarding Yellow Fever?
|
Eliminate sites where Aedes mosquitoes grow; Vaccination (live attenuated vaccine) – treatment is supportive only
|
|
|
How is the diagnosis of Yellow Fever made?
|
Patient history; ELISA, RT-PCR, FA, culture
|
|
|
What describes the symptomatology of Yellow Fever?
|
Incubation period: 3-6 days; Acute phase: fever, HA, muscle aches (back, knees), n/v, loss of appetite, dizziness, red eyes/face/tongue; Toxic phase (15% of cases): jaundice, abdominal pain, vomiting (sometime blood), decreased urination, bleeding from nose/mouth/eyes, arrhythmias, liver/kidney failure, brain dysfunction (incl. delirium, seizures, coma), death (20-50% of toxic cases)
|
|
|
What are the outcomes for WNV?
|
Younger age is more predictive of full recovery than is initial illness severity; WN meningitis mortality is almost none, while WN encephalitis mortality is 15-25%; With WN flaccid paralysis some people have almost complete recovery, others continued weakness
|
|
|
What characterizes Tick-borne encephalitis?
|
Transmitted by the Deer tick; not endemic in the US, prevalent in Europe and Asia
|
|
|
What characterizes St. Louis encephalitis?
|
Transmitted by the Culex mosquito throughout the US; transmission is from infected birds to humans; most have a mild case, few develop encephalitis; rare disease
|
|
|
What are the alphaviruses?
|
Eastern equine encephalitis (EEE), Western equine encephalitis (WEE), Venezuelan equine encephalitis (VEE)
|
|
|
What characterizes WEE?
|
Most cases are unapparent; natural cycle involves birds/mosquitoes; inactivated vaccine available
|
|
|
What characterizes EEE?
|
Rare but deadly disease (if disease progresses to encephalitis, mortality is 90%); there is a vaccine available for horses and laboratory workers
|
|
|
What can cause impairment of the CMI (Cell-Mediated Immunity)?
|
Certain medications (immunosuppressives, corticosteroids); AIDS; extremes of age; lymphomas; malnutrition
|
|
|
What infections are associated with asplenia?
|
Certain encapsulated bacteria: *Streptococcus pneumoniae (MOST IMPORTANT), Haemophilus influenzae
Other bacteria: Neisseria meningitidis, Salmonella species, C. canimorsus (dog contact) Other infections worse after splenectomy: Babesiosis; Ehrlichiosis |
|
|
What are examples of common humoral deficiencies? What is the common presentation?
|
Primary Immunoglobulin deficiencies
Genetic, X-linked agammaglobulinemia (rare; severe) Acquired: Selective IgA deficiency - most common humoral defect in population at large Common Variable Immunodeficiency – decreased levels of IgG, IgM, IgA *Both acquired disorders may present with recurrent sinus and pulmonary infections |
|
|
Why do bacteria produce a capsule?
|
Protection from PMNs (granulocytes)
|
|
|
What type of organisms does humoral immunity provide protection?
|
Important in protection against infection w/ bacteria, esp. encapsulated ones; Strep. pneumoniae, H. influenzae
|
|
|
What is a common manifestation of a Listeria (g + rod) infection?
|
Meningitis
|
|
|
What infections are associated with defects in the CMI?
|
Bacteria (esp. intracellular pathogens): Mycobacteria, Legionellae, Listeria, Nocardia, Salmonella;
Fungi: Cryptococcus, Histoplasma, Pneumocystis jirovecii Viruses: Cytomegalovirus, Herpes simplex, Varicella-zoster virus Parasites: Toxoplasma gondii (found in brain, pathology exclusive to CMI defect) |
|
|
What is the most common defect of neutrophils?
|
Neutropenia (absolute count <1000)
|
|
|
What infection is associated with complement deficiencies (esp. C5-C9)?
|
Neisseria meningitidis (!) (possibly Neisseria gonorrhea)
|
|
|
What infections are associated with neutropenia?
|
Mainly organisms of natural flora - Gram negative enteric rods, Pseudomonas aeruginosa, Staph & Strep species, Candida (esp. retina, liver/spleen), Aspergillus
|
|
|
What other disease states are associated with lower immunoglobulin levels or function?
|
CLL; multiple myeloma; nephrotic syndrome; splenectomized patients; AIDS
|
|
|
What diagnostic test is used to ID Cryptococcus neoformans?
|
India ink, Crypto Antigen test
|
|
|
Which causes of meningitis present chronically w/ low CSF glucose?
|
Cryptococcus neoformans, other fungi, Mycobacterium tuberculosis,
Less likely: Listeria monocytogenes (but course is probably too long) Viruses such as CMV, HSV, VZV etc, (CSF glucose usually only slightly depressed in viral CNS infection) |
|
|
What diagnostic test should be used in a patient w/ recurrent sinus/pulmonary infections? Which organisms involved? Possible diagnosis? Tx?
|
CBC, differential and platelet count, PPD and controls (screening test for cell-mediated immunity), quantitative serum immunoglobulin levels, HIV antibody test, CD4 count.
Pyogenic bacteria especially encapsulated species (ex. Streptococcus pneumoniae, Haemophilus influenzae) Dx - Common Variable Immunodeficiency - they won’t respond to vaccines Tx - IV Ig |
|
|
Does undernutrition occur solely in poor populations?
|
No – it occurs in affluent as well as poor populations
|
|
|
What are two examples of PEM?
|
Marasmus, Kwashiorkor
|
|
|
What tests are used to assess PEM in each of the two functional protein compartments?
|
Somatic compartment: body weight and height vs. standard, loss of fat (reduced thickness of skin folds), loss of muscle (reduced mid-arm circumference)
Visceral compartment: measure of visceral proteins (albumin, prealbumin, transferin) |
|
|
What are the two functional protein compartments and what diseases are associated with PEM in each?
|
Somatic compartment (skeletal muscles): more affected by calorie deficiency --> marasmus
Visceral compartment (liver): more affected by protein deficiency --> kwashiorkor |
|
|
What are some common causes of malnutrition?
|
Ignorance/poverty: PEM in elderly/homeless/poor, unrecognized increased nutritional needs in infants/adolescents/pregnant women
Chronic alcoholism: PEM, more risk for vitamin deficiencies (thiamine, pyridoxine, folate, vitamin A), multifactorial causes (poor diet, absorption, high loss, etc) Acute/chronic illnesses: elevated BMR (e.g. burn patients), increased requirement for all nutrients Self-imposed dietary restriction: anorexia, bulimia, body-image-concerned, fearful of CV disease |
|
|
What are some uncommon causes of malnutrition?
|
Malabsorption syndromes, genetic diseases, drug therapies that interfere with uptake of a specific nutrients, total IV nutrition
|
|
|
Where in the world is protein-energy malnutrition (PEM) most common?
|
In 3rd world (affects up to 25% of children) and developing countries, whereas overnutrition is prevalent in industrialized nations
|
|
|
What body in the US is responsible for setting the daily allowances for proteins, vitamin, and minerals for healthy adults?
|
National Research Council
|
|
|
What is secondary malnutrition?
|
Malnutrition despite nutrient supply deemed to be adequate for a normal person – this can be due to various reasons: malabsorption, impaired use/storage of nutrients, excess loss of nutrients, increased need for nutrients (burn patients)
|
|
|
What is an adequate diet?
|
One that provide energy in form of carbohydrates/fat/proteins, essential AA, essential fatty acids, vitamins, and minerals – any diet missing one or more of these components in adequate amounts is called “primary malnutrition”
|
|
|
What clinical findings are associated with Kwashiorkor?
|
Enlarged and fatty liver due to lack of lipoprotein production in liver to mobilize fats
“Flaky paint” appearance of skin with alternating areas of hypo/hyperpigmentation and desquamation |
|
|
What characterizes Anorexia nervosa and Bulimia nervos?
|
Anorexia nervosa: Self-induced starvation, marked weight loss
Builima nervosa: binge-eating followed by self-induced vomiting |
|
|
What changes occur similarly in both Kwashiorkor and Marasmus?
|
Hypoplastic bone marrow --> reduced RBCs --> anemia (hypochromic microcytic, mixed micro/macrocytic with folate deficiency)
|
|
|
What changes occur mostly in Kwashiorkor and rarely in Marasmus?
|
Reversible small bowel mucosal atrophy, loss of villi and digestive enzymes (e.g. disaccaridase deficiency --> leading to milk intolerance in babies), Liver enlargement due to fatty liver
|
|
|
How does BMR change in chronic starvation and cachexia?
|
Decreases in chronic starvation, increases in cachexia due to cytokines produced during sepsis or by tumors (e.g. TNF)
|
|
|
What is cachexia?
|
Severe form of Marasmus seen in chronic illnesses (lung/GI, cancer) – associated with loss of SC fat, wasting of quadriceps/deltoid muscles, and ankle and sacral edema
|
|
|
What is a clinical setting in which Kwashiorkor is seen?
|
Acute, catabolic illness (severe trauma, burn, sepsis), develops over weeks, leads to low serum albumin (<2.8 gm/dL), edema, easy pluckable hair (normal fat and muscle)
|
|
|
What characterizes Marasmus?
|
Reduced caloric intake, >60% reduction in body weight with extremities emaciated, head appearing too large, and deficiency in immune system – associated with loss of somatic protein, muscle mass, SC fat; visceral protein depletion is slight (serum albumin normal or slightly reduced)
|
|
|
What characterizes Kwashiorkor?
|
More severe than Marasmus, caloric intake is reduced and consists of carbs, protein intake is severely reduced --> hypoalbuminemia --> edema, depletion of visceral protein, 60-80% of normal weight, relative sparing of SC fat and muscle mass
|
|
|
What is a clinical setting in which Marasmus is seen?
|
Chronic illness (chronic lung/GI disease, cancer), develops over months, leads to loss of weight/muscle/SC fat with normal or mildly reduced serum proteins
|
|
|
What are measures of fat accumulation?
|
BMI (kg per square meter): underweight: < 18.5, normal: 18.5-24.9, overweight: 25.0-29.9, obese: ≥ 30
Skin fold measurements, body circumferences (ratio of waist to hip) |
|
|
What are effects of diet on systemic diseases?
|
Low sodium intake: reduces HTN – dietary fiber: reduces colon diverticulosis
Omege-3 fatty acids in diet: significantly reduces fatal MI Caloric restriction: increases lifespan in experimental animals |
|
|
What are some obesity-related conditions?
|
Insulin resistance/hyperinsulinemia: type II diabetes, improves with weight loss
HTN: risk increases with weight in previously normotensive individuals, may be due to hyperinsulinemia Non-alcoholic steatohepatitis, cholelithiasis (gallstones), osteoarthritis, venous thrombosis, ischemic stroke Hypoventilation syndrome (Pickwick syndrome): day and night hypersomnolence, sleep apnea, polycythemia, right-sided heart failure Metabolic syndrome (syndrome X): abdominal obesity, insulin resistance, hyperTAGemia, low serum HDL, HTN, increased risk of CAD (!) |
|
|
What system in the body regulates energy intake and expenditure?
|
Lipostat or internal set point: keeps body weight in narrow range for years, regulation is done by hormones
Ghrelin (short term control): secreted by stomach, increases before meals, decreases when full Leptin (long term control): produced by fat cells, inhibits anabolism, triggers catabolism, net effect is reduce food intake and increase energy expenditure (!) *** |
|
|
What shape of central obesity is associated with higher risk of high BMI morbidities?
|
Apple-shape with abdominal and chest fat confers higher risk morbidities (pear –shape with fat in thighs/hips confers lower risk)
|
|
|
What are two general positions of fat accumulation in people with high BMI?
|
Central (visceral), subcutaneous
|
|
|
What morbidities are associated with high BMI?
|
Beginning at BMI of 25, there is increased prevalence of diabetes, HTN, CAD, osteoarthritis
|
|
|
What are the clinical findings in Anorexia nervosa?
|
Similar to severe REM plus endocrine effects: amenorrhea (low GRH), low thyroid hormone (cold intolerance, bradycardia, constipation, dry skin, fine/pale hair), decreased bone density (low estrogen, mimicking post-menopausal osteoporosis), lymphopenia, anemia, hypoalbuminemia
|
|
|
What are the clinical findings in Bulimia nervosa?
|
Less amenorrhea (<50%), normal weight, major complications due to vomiting (electrolyte imbalance, hypokalemia, arrhythmias – aspiration – rupture of esophagus)
|
|
|
What is leading cause of death in Anorexia nervosa?
|
Cardiac arrhythmia and sudden death due to hypokalemia
|
|
|
What is the consensus on the effect of diet on carcinogenesis?
|
There is no definite proof that diet can cause or protect against cancer (!) ***
|
|
|
What aspects of a clinical history suggest pesticide illness?
|
Multiple cases with similar symptoms and an exposure history; History of chemical application (home or office); Accidental ingestion (esp. children); Suicide/homicide attempts
|
|
|
What characterizes rodenticides?
|
In general, they have no target selectivity (except for behavioral differences between various animas), some include emetics so that cats/dogs/babies will vomit, but rodents won’t (lack reflex)
|
|
|
What are examples of fungicides that are toxic to humans?
|
Thiocarbamates (e.g. Zineb): sensitizer in skin patch test, dermatitis, Antabuse-like reaction;
Phtalimide derivatives (e.g. Captan): sensitizes, dermatitis; Substituted aromatics (e.g. Chlorothalonil, Hexachlorobenzene): sensitizes, dermatitis; Inorganic (e.g. elemental sulfur): skin irritant |
|
|
What characterizes the bipyridyl herbicides?
|
Broad-spectrum herbicides; Paraquat: highly toxic, use restricted to registered pesticide applicators, overexposure results in lung toxicity (active transport into the lung --> produces ROS); Diquat: overexposure results in GI/renal toxicity
|
|
|
What characterizes the chlorophenoxyacetic acids?
|
Used for broadleaf plants; Key compound is 2,4-dichlorophenoxyacetic acid (2,4-D); Overexposure causes muscle weakness, aching and tenderness, renal failure
|
|
|
What characterizes herbicides?
|
Chemicals that kill plants; in general, they have low toxicity (except for some dermal irritation); Examples that do affect humans incl. chlorophenoxyacetic acids and bipyridyl herbicides
|
|
|
What are the signs/symptoms of pesticide illness?
|
Non-specific s/s: rash, flu-like symptoms (dizziness, malaise, respiratory tract irritation), GI symptoms, seizures, odor-related effects (not toxicological effects of active ingredient)
|
|
|
What pesticides or metabolites can be detected in the general population due to exposure?
|
Organophosphates: parathion, chlorpyrifos; Organochlorines: DDT-related compounds;
Pyrethrin; Herbicides: 2,4-D; Pest repellents: DEET |
|
|
What substances are found in pesticide formulations?
|
Technical grade chemical (the active ingredient); Adjuvants/synergists; “Inert” ingredients (does not mean biologically inactive, just means that they are not the active ingredient)
|
|
|
What is the US EPA toxicity classification?
|
Class I: danger, fatal if ingested, corneal opacity, corrosive to skin;
Class II: warning, may be fatal if ingested, reversible corneal opacity, severe skin irritation; Class III: caution, harmful if ingested, no corneal opacity, moderate skin irritation; Class IV: caution, may be harmful if ingested, no eye irritation, mild/no skin irritation |
|
|
What are examples of other rodenticides?
|
Coumarins/Indandiones: decrease synthesis of clotting factors, increase capillary permeability, these incl. warfarin, brodifacoum/difenacoum (“superwarfarins”), chlorphacinone (Indandione);
Zinc phosphide: causes pulmonary edema in dust form, when ingested a phosphine is formed and absorbed in the gut leading to n/v, hepatic failure, hypoglycemia, renal tubular damage, cardiomyopathy, ventricular arrhythmias |
|
|
What characterizes the pyrethrin and pyrethroids?
|
Products of chrysanthemums, natural products (contact allergies, asthma, anaphylactic reactions); Frequent cause of pesticide-induced symptomatic illness (generally not fatal in the US);
Examples: pyrethrin, fenvalerate, permethrin; MoA: alters ion flux across neutral membranes; Overexposure: neuronal hyperactivity, incoordination, tremors with hyperthermia, seizures; Treatment: Diazepam |
|
|
What are the signs/symptoms of overexposure to organochlorine insecticides and what is the treatment?
|
Overexposure: paresthesias (esp. of tongue/face/lips), apprehension, irritability (highly responsive to stimuli), tremors, convulsion; Treatment: Diazepam, Phenobarbital
|
|
|
What is the mechanism of action of organochlorine insecticides?
|
Interfere with ion movements across neutral tissue membranes (GABA related)
|
|
|
What characterizes the organochlorine insecticides?
|
Examples incl. dichlorodiphenylethanes (DDT, dicofol [Kelthane]) and chlorinated benzenes (lindane); Most are banned because of environmental problems (resistant to degradation in environment, bioaccumulation, DDT has estrogenic effects/impaired fertility/eggshell thinning)
|
|
|
What characterizes the insecticides?
|
Highly lipid soluble, metabolism via bioactivation/inactivation, generally neurotoxic;
Classes: organochlorines, pyrethrins/pyrethroids, cholinesterase inhibitors (carbamates, organophosphates) |
|
|
What characterizes DEET (diethyltoluamide)?
|
Common pesticide implicated in symptomatic illness; Causes skin irritation to blistering and erosion (esp. in occluded areas); When systemically absorbed, causes toxic encephalopathy (HA, restlessness, irritability, incoherent speech, ataxia, loss of consciousness, seizures) – children should use DEET concentrations below 10% (discontinue with emotional/behavioral change)
|
|
|
What are examples of rodenticides that function as convulsants?
|
Compound 1080: blocks energy metabolism, induces metabolic acidosis, shock, arrhythmias, neurotoxicity (violent tonic-clonic convulsions); Strychnine: direct convulsant
|
|
|
What signs/symptoms are seen in agricultural workers exposed to cholinesterase inhibitors?
|
GI distress (n/v), miosis, urinary frequency, sweating, weakness, bradycardia
|
|
|
By what mechanism does irreversible inhibition of AChE come about?
|
The serine residue on AChE attacks phosphate on the organophosphate molecule --> the OP becomes attached to the serine residue and does not undergo hydrolysis to return to the original state (i.e. AChE is stuck with the OP and cannot perform any more reactions)
|
|
|
What is the mechanism of ACh hydrolysis by acetylcholinesterase?
|
ACh docks inside AChE --> a serine residue on AChE attacks the acetyl portion of ACh, causing it to become attached to the serine residue --> water then attacks the acetyl molecule on serine, causing it to be released, and returning AChE to its original state
|
|
|
What long-term effects are seen in patients exposed to high levels of organophosphates?
|
Long-term effects: fasciculations, weakness, impaired memory/concentration, emotional lability;
Intermediate syndrome (1-4 days after acute): muscle weakness, respiratory distress; Chronic toxicity (OP-induced delayed neuropathy, OPIDN): axonal/myelin degeneration, occurs with some non-pesticide organophosphates, is due to neurotoxic esterase inhibition |
|
|
What is the treatment for cholinesterase inhibitor overexposure?
|
Atropine: competes with ACh at the receptor, dose must be repeated every 10-30 minutes;
2-PAM: reactivates enzyme (for organophosphates only), adverse effects incl. arrhythmias/HTN |
|
|
What can be done for a lab diagnosis of exposure to cholinesterase inhibitors?
|
Blood tests for “true”/acetylcholinesterase (RBC) or “pseudo”/butyrylcholinesterase (plasma);
Urine metabolites; Foliage and clothing pesticide residues – diagnosis is primarily clinical |
|
|
What signs/symptoms are seen in children exposed to cholinesterase inhibitors?
|
Cholinergic signs less pronounced; Can see seizures, mental status changes, flaccid muscle weakness
|
|
|
What characterizes the carbamate insecticides?
|
Examples incl. carbaryl (Sevin); MoA: reversible inhibition of acetylcholinesterase; Overexposure: same symptoms as organophosphates; Overexposure treatment: Atropine
|
|
|
What are the signs/symptoms of cholinesterase inhibition?
|
All are due to excess acetylcholine (parasympathetic, somatic, CNS stimulation) –
Eyes: miosis, blurred vision; Glands: tearing, salivation, sweating, pulmonary edema; Heart: bradycardia, tachycardia; Smooth muscle: bronchoconstriction, vomiting, diarrhea, urination; Skeletal muscle: fasciculations, twitching, cramps, weakness, paralysis; CNS: HA, malaise, anxiety, confusion, impaired memory, convulsions, coma |
|
|
What characterizes the organophosphate (OP) insecticides?
|
Number one pesticide implicated in fatal poisonings; Examples incl. parathion, malathion;
MoA: inhibition of acetylcholinesterase (can be become irreversible) |
|
|
What is meant by the “aging” of AChE?
|
The irreversible inhibition of AChE (the pace at which “aging” occurs depends on the chemistry of the AChE inhibitor)
|
|
|
What are the signs/symptoms for skin exposure to a nerve agent?
|
Skin sweating at the site, muscle fasciculation in the area, systemic GI effects (30min-18hrs later)
|
|
|
What is the treatment for exposure to nerve agents (Sarin, Soman, or VX)?
|
Atropine, 2-PAM, Diazepam, Atropine (topical for eye pain from miosis)
|
|
|
What are common effects that occur with exposure to Sarin, Soman, or VX?
|
Runny nose, watery eyes, miosis, eye pain, blurred vision, drooling, excessive sweating, cough, chest tightness, tachypnea, d/n/v, increased urination, confusion, drowsiness, weakness, HA, slow or fast HR, abnormally low or high BP – with vapor exposure, effects can appear within seconds; with liquid exposure, effects can appear within minutes to up to 18hrs; latex/polyvinyl gloves are not a good barrier for handling these agents (use nitrile/neoprene gloves)
|
|
|
What characterizes VX?
|
It is an oily liquid, amber in color; it evaporates very slowly, but contact is considered lethal; it is odorless and tasteless
|
|
|
What characterizes Sarin nerve gas?
|
The most volatile of the nerve agents; clear, colorless, and tasteless liquid that has no odor –
Soman is a nerve gas of the same class that causes “aging” within 2 minutes of exposure |
|
|
How does 2-PAM work?
|
It attacks the phosphate group that is bound to the inactivated serine residue on AChE, and causes AChE to return to its original state, thus restoring its functionality – treatment must be given before the “aging” reaction takes place
|
|
|
What is the role of metabolism with solvent toxicity?
|
Acute effects on CNS are due to parent compound; Delayed toxicities are associated with metabolites formed in affected tissue
|
|
|
What characterizes hexacarbon neuropathy?
|
Caused by the metabolite of hexane and methyl-N-butyl ketone: 2,5-hexanedione; this compound forms a pyrrole with lysine, which then causes cross-linking of neurofibrils, thus inhibiting neurofibril transport; this leads to swelling of axons as proteins accumulate
|
|
|
What are the aromatic hydrocarbons that cause solvent toxicity?
|
Benzene: used for high-speed printing/latex rubber coating/anti-knock, causes bone marrow toxicity (aplastic anemia/leukemia), metabolites incl phenol/catechol/hydroquinone/benzoquinone, hydroquinone and benzoquinone are involved in a redox cycle and generate ROS;
Toluene: causes CNS depression, atrophy (with high exposure), ototoxicity, liver/kidney toxicity, reproductive toxicity (possible) – both are metabolized by CYP2E1 |
|
|
What other signs/symptoms are seen with solvent toxicity?
|
Toxic peripheral neuropathy (axonopathy), hearing loss, hematological effects (leukemia, aplastic anemia), renal effects (tubular toxicity, cancer), asthma
|
|
|
What characterizes the chronic symptomatology of solvent-related diseases?
|
Chronic toxic encephalopathy – Mild/type 1: fatigue, memory impairment, irritability, difficulty concentrating, mild mood disturbance; Mild chronic/type 2: sustained personality changes, impaired intellectual function/learning capacity); Severe chronic/type 3 (Painter’s syndrome/ psycho-organic syndrome): global deterioration, cerebellar/cerebral atrophy in abusers
|
|
|
What characterizes the acute symptomatology of solvent-related diseases?
|
Acute intoxication: CNS depression (sedation, narcosis, anesthesia);
|
|
|
Which CYP enzyme is involved in the formation of toxic metabolites from solvents?
|
CYP2E1 (induced by alcohol, poorly controlled diabetes, and fasting)
|
|
|
What are important properties of the various solvents?
|
Polar vs nonpolar, hydrophilic vs lipophilic, volatility, flammability – in terms of toxicity, nonpolar, lipophilic solvents have the greatest concern
|
|
|
What aspects of solvent exposure are regulated?
|
Time weighted average (TWA) of solvent exposure (i.e. the total amount over a period of time);
Acceptable ceiling concentration/short-term exposure limit (i.e. the maximum peak concentration) |
|
|
What are solvents used for?
|
Degreasing agents, vehicle for coatings, chemical manufacturing, (also with manicures)
|
|
|
What characterizes carbon disulfide (CS2)?
|
Used for the production of rayon/cellophane; Exposure effects: organic brain damage (memory loss, mood changes), hearing loss, visual disturbances, distal neuropathy/axonopathy (neurofilament cross-linking), atherosclerosis
|
|
|
What characterizes the glycols (e.g. ethylene glycol, propylene glycol)?
|
Ethylene glycol: used as antifreeze, metabolized to oxalic acid, causes renal toxicity (oxalate stones), treatment is fomepizole; Propylene glycol: used as a drug vehicle, metabolized to lactate
|
|
|
What characterizes the aliphatic alcohols (ethanol, methanol)?
|
Ethanol: anesthetic (poor therapeutic index), induces CYP2E1, metabolized to acetaldehyde and acetic acid; Methanol: causes CNS depression, blindness, acidosis, metabolized to formaldehyde and formic acid (builds up), treated by inhibiting its metabolism (i.e. via ethanol or fomepizole – fomepizole does not have the CNS depressant effects of ethanol)
|
|
|
What characterizes the chlorinated ethane/ethylene derivatives (e.g. trichloroethylene)?
|
Used as a degreasing agent (dry cleaning) and anesthetic (formerly); Exposure effects: myocardial sensitization, increases sensitivity to ethanol, renal cancer, impaired intrauterine growth, cardiac teratogenesis; Metabolized by CYP enzymes and alcohol dehydrogenase (competitive inhibition); toxic metabolites are formed through CYP enzymes and through glutathione conjugation (in the kidney)
|
|
|
What characterizes the halomethanes: chloroform, carbon tetrachloride, methylene chloride?
|
CHCl3, CCl4: water disinfection by-products, used in pharmaceutical industry, causes centrilobular necrosis, both undergo bioactivation by P450, toxicity increased by fasting/alcohol/poorly controlled DM (i.e. induction of CYP2E1), they sensitize heart muscle to arrhythmogenic effects of endogenous epinephrine; CH2Cl2: paint stripper/degreasing agent/former fire extinguisher/ decaffeinating agent (not anymore), metabolized to CO, previous exposure standard was based on minimizing carboxyhemoglobin formation but currently based on carcinogenicity
|
|
|
What characterizes dimethylformamide – HCON(CH3)2?
|
Used for acrylic fiber spinning, chemical manufacture, coatings/adhesives, textile dyes, printing;
Exposure effects: easily absorbed across skin/lungs, HA, dizziness, abd. pain, n/v, constipation, liver damage, alcohol intolerance, possibly carcinogenic |
|
|
What are the most common side effects associated with the use of NSAIDs?
|
GI upset (can cause ulcers, most common); Renal problems (due to effects on PG’s then can change intrarenal blood flow & lead into kidney failure); Lung problems (causes exacerbation of asthma)
|
|
|
Beyond steroids and NSAIDs, What is the most commonly prescribed drug for the treatment of RA in the US?
|
Methotrexate
|
|
|
How many people with RA take steroids for treatment?
|
About 50% of pts take a small dose of prednisone every day
|
|
|
What is the most severe side effect of steroids?
|
Really lowers resistance to infections (also weight gain, diabetes, osteoporosis, cataracts)
|
|
|
What is the safest drug known for the treatment of RA?
|
Hydroxychloroquine
|
|
|
What antimalarial agent is used as a treatment of RA?
|
Hydroxychloroquine, MOA is unknown
|
|
|
What Gold historically used in treating?
|
RA, given as shots; Example of the 1st controlled trial of a drug in the treatment of a disease that showed efficacy, done in England before WWII
|
|
|
What are the symptoms of Rheumatoid Arthritis?
|
Morning stiffness (most prominent), Joint swelling in wrists, MCP & PIP joints, & Joint Pain
|
|
|
What do all NSAIDs how in common?
|
They are all prostaglandin synthetase inhibitors, they work by interfering w/ the synthesis of intermediaries of metabolism that are all prostaglandin or prostaglandin-related, they all have the same efficacy & very similar side effect profiles
|
|
|
How does essentially everyone with RA begin treatment?
|
NSAIDs (Aspirin, Ibuprofen, Naprosyn, Naproxen,, Tolmine Sodium, Indomethacin)
|
|
|
What is the Theoretical advantage of giving Etanercept & Adalimumab over Infliximab?
|
You do not having this crossing species line issues because they are all human vs Infliximab which is part human part mouse chimeric antibody, Theoretically the immunological rxn in the pts body to receiving these injections will be less with Etanercept & Adalimumab than with Infliximab
|
|
|
What drugs can cause reactivation of TB?
|
Biologics, particularly Infliximab, you need to check the pt’s PPD before starting treatment
|
|
|
What are the two main side effects apart from the immunologic factors of biologics?
|
Site reaction where the shot is given (most common)May be an increased incidence of cancers (lymphomas, adenocarcinomas), Increased Incidence of infections, demyelination of the CNS
|
|
|
What receptor fusion protein is used for the treatment RA?
|
Etanercept (Enbrel), It is a receptor for TNF- α that you give by injections and it circulates in their circulation, & soaks up the TNF-α
|
|
|
What Human-mouse chimera antibody is used for the treatment RA?
|
Human-mouse chimera antibody to TNF- α, Anti-TNF- α antibody
|
|
|
What Human Monoclonal Antibody is used for the treatment RA?
|
Adalimumab (Humira), it is a circulating antibody to TNF- α
|
|
|
What are the biologics used for the treatment of RA?
|
Etanercept, Adalimumab, Infliximab
|
|
|
What describes the MOA & side effects of MTX?
|
MOA: It is a folic acid inhibitor, it inhibits an enzyme called Dihydrofolate Reductase; Side Effects: mouth sores, lowered blood counts, can make your hair fall out, cirrhosis
|
|
|
What are the antimetabolites used in the treatment of RA?
|
Methotrexate (MTX), Azathioprine (AZA)
|
|
|
What is the rare side effect of MTX that indicates a CI for what?
|
Can cause micronodular cirrhosis of the liver (looks like the type of cirrhosis seen in ppl who drink), Alcohol is CI
|
|
|
How many adults in the US have metabolic syndrome?
|
1 out of 5 adults, 40% of ppl over 60 yo
|
|
|
What is the 1st step in the Metabolic Syndrome Treatment Algorithm?
|
Lifestyle Intervention (diet, exercise, smoking cessation) for at least 3-6 months
|
|
|
What elements are involved in Metabolic Syndrome (a.k.a. Insulin resistant state)?
|
Glucose intolerance, Dyslipidemia, HTN, Hypercoagulability, Hyperinsulinemia; they can all lead to cardiovascular disease (intervene to prevent)
|
|
|
What are the associated abnormalities of Metabolic Syndrome?
|
NASH; Obstructive sleep apnea; PCOS; Increased inflammatory markers/cytokines (IL-6, CRP, PAI-1)
|
|
|
What defines Metabolic Syndrome in Children (IDF)?
|
Criteria for children aged 10-16
(1) Obesity - > 90th percentile (2) TG > 150mg/dl; (3) HDL-C < 40mg/dl; (4) BP > 130/85; (5) Glucose > 100mg/dl or T2DM (OGTT) |
|
|
What defines the metabolic syndrome in Adults?
|
Need 3 out of 5 to meet criteria:
(1) Abdominal obesity, waist circumference in M >102 cm (40 in) & in F >88 cm (35 in); (2) Serum triglycerides ≥150 mg/dL (1.7 mmol/L) or drug treatment for elevated triglycerides; (3) Serum HDL cholesterol <40 mg/dL (1 mmol/L) in M <50 mg/dL (1.3 mmol/L) in F or drug treatment for low HDL-C; (4) BP ≥130/85 mmHg or drug treatment for elevated BP; (5) Fasting plasma glucose (FPG) ≥100 mg/dL (5.6 mmol/L) or drug treatment for elevated blood gl |
|
|
What A1C level is considered to be diagnostic of diabetes and prediabetes?
|
Diabetic: A1C > 6.5;
Prediabetic: A1C 5.7-6.5 |
|
|
What are the treatment goals of metabolic syndrome after lifestyle modifications alone have failed?
|
In addition to lifestyle modification:
(1) Achieve target A1C goal (< 7%); (2) Avoid hypoglycemia; (3) Avoid weight gain; (4) Improve BP and lipid control; (5) Optimize patient adherence* |
|
|
What are the triad of defects seen in type 2 DM?
|
(1) Defective insulin secretion (beta-cell dysfunction); (2) Insulin resistance (fat and muscle); (3) Increased hepatic glucose output
|
|
|
What is the drug of choice at time of Dx of DM-2?
|
Lifestyle modification and Metformin (target is to decrease hepatic glucose output) unless there is an indication not to give it
|
|
|
What is the best drug to start for HTN in a patient with DM?
|
Angiotensin converting enzyme inhibitors or Angiotensin converting enzyme blockers
|
|
|
What classifies the Critical Biological Agents: Category A (CDC) and what pathogens are included?
|
Pathogens that pose greatest risk to national security:
(1) Can be easily disseminated OR transmitted person-to-person; (2) Cause high mortality with potential for major public health impact; Bacillus anthracis (anthrax); Variola major (smallpox); Clostridium botulinum (botulism); Yersinia pestis (plague); Francisella tularensis (tularemia); Hemorrhagic fever viruses |
|
|
What are the virulence factors of B. anthracis that cause disease?
|
Organism produces antiphagocytic capsule & edema factor (EF), lethal factor (LF) & protective antigen (PA) --> produce Edema Toxin & Lethal toxin --> necrosis of lymphatic tissues ---> causes release of large numbers of B. anthracis into bloodstream --> Overwhelming septicemia rapidly occurs
|
|
|
How does Inhalation Anthrax replicate within the human host?
|
Exposure to necessary inoculum, LD50 estimated to be 8,000-10,000 inhaled spores, Uptake of spores by pulmonary macrophages which carry spores to tracheobronchial or mediastinal lymph nodes, Spores vegetate --> replicating bacteria
|
|
|
What are the 3 types of Clinical Manifestations of Anthrax?
|
Inhalational (most serious);
Cutaneous (most common in natural form); Gastrointestinal (least likely) |
|
|
What is Bacillus anthracic and how does it cause disease?
|
Aerobic, gram positive spore-forming, non-motile, non-hemolytic species, it is acquired usually by contact with infected animals or their hides, Spores can survive for decades and they germinate when enter environment rich in nutrients
|
|
|
What organisms are Critical Biological Agents in Category C (potentially a threat) of bioterrorism?
|
Hantavirus;
Yellow Fever; Multi-drug resistant TB; Genetically-altered microbes |
|
|
What organisms are Critical Biological Agents in Category B (Moderate Risk) of bioterrorism?
|
Coxiella burnetti (Q fever);
Brucella species (brucellosis); Burkholderia mallei (glanders); Alphaviruses (VEE, others); Toxins: ricin, Staph. Enterotoxin B; Food and waterborne pathogens |
|
|
What is the definition of bioterrorism?
|
“Those occurrences, (deliberate), that result in human suffering and damage from exposure to biological agents”
|
|
|
What are the Epidemiological Features of Possible Bioterrorism Attacks?
|
Clusters of pts from single locale; Rapidly increasing disease incidence; Endemic disease rapidly emerging at unusual time or pattern; Any pt presenting w/ a disease that is uncommon and has bioterrorism potential
|
|
|
What is the incubation period of Inhalational Anthrax & what develops after incubation?
|
Incubation period of 1-16 days, but cases may arise later, up to 60 days; After incubation a nonspecific flu-like illness develops (Fever, myalgia, HA, nonproductive cough, mild chest discomfort, n/v)
|
|
|
What organisms are Critical Biological Agents in Category C (potentially a threat) of bioterrorism?
|
Hantavirus;
Yellow Fever; Multi-drug resistant TB; Genetically-altered microbes |
|
|
What is the definition of bioterrorism?
|
“Those occurrences, (deliberate), that result in human suffering and damage from exposure to biological agents”
|
|
|
What are the clinical features of Inhalational Anthrax?
|
High fever, dyspnea, stridor, then rapid respiratory failure, shock; Hemorrhagic meningitis (in 50% cases) may occur late; Mortality > 90% (historically)
|
|
|
What is the emperic and prophylactic ABX treatment?
|
Emperic: Ciprofloxacin IV or Doxycycline IV for 60 days, in adults AND children; Prophylactic: PO Doxycycline or Ciprofloxacin
|
|
|
How is Gastrointestinal Anthrax?
|
Follows ingestion of insufficiently cooked contaminated meat, presents severe abdominal distress, followed by fever, signs of septicemia, Case-fatality rate 25-60%
|
|
|
What pathognomic of cutaneous anthrax lesion?
|
Starts as papule, then vesicle, then depressed black eschar
|
|
|
What infection disease is of major concern due to a 30% case fatality rate, physically disfiguring, no treatment available, very communicable from person-to-person, & many susceptible people today?
|
Smallpox, an acute viral illness caused by the variola virus
|
|
|
What are characterized the transmission and clinical manifestations of Smallpox?
|
Spreads by aerosol or droplet nuclei from oropharynx (Contaminated clothing, linen could also spread virus; Incubation period 7-17 d; high fever, malaise, prostration with headache, backache, Severe abdominal pain, delirium)
|
|
|
What other rashes can mimic smallpox and what should you do?
|
Chicken pox, monkey pox, disseminated cutaneous herpes simplex; Then send specimens to State lab, CDC: PCR of tissue available; Observing typical virions by electron microscopy, Viral antigens, culture
|
|
|
How do the lesions of smallpox differ than those of chickenpox?
|
Smallpox: Maculopapular rash appears 1st on mucosa of mouth, pharynx, face & forearms, spreading to trunk & legs; W/in 1-2 days, rash becomes *vesicular*then pustular (tense, deep in dermis), Crusts form d 8-9, separate, then scar, *Lesions develop synchronously**
(vs Chickenpox which have lesions in different stages of development on the same pt) |
|
|
Why is the smallpox vaccine contraindication in pre-exposure setting to pts who are immunocompromised, have certain skin diseases, or are pregnant?
|
Vaccinia can spread through out the skin & cause disseminated Vaccinia
|
|
|
What is the unique feature of the post-exposure smallpox vaccine that is not seen in other infectious diseases?
|
if vaccinated them w/in 3 days after exposure, you can prevent smallpox disease
|
|
|
What toxin inhibits release of acetylcholine resulting in flaccid paralysis?
|
Botulinum toxin, potent neurotoxin produced by Clostridium botulinum (anaerobic gram positive bacillus), Spores present in soil and marine sediment worldwide
|
|
|
What is suspected in a pt that presents as Responsive w/ absence of fever, Symmetrical cranial neuropathies, Blurred vision, diplopia, symmetrical descending weakness in a proximal to distal pattern & has no sensory deficits?
|
Botulism
|
|
|
What is the incubation period for Botulism and how is it managed?
|
Foodborne: 12-36 hrs after exposure;
Inhalational: 24-72 hrs exposure; Supportive therapy & Trivalent botulinum antitoxin in the inhalational bioterrorism form |
|
|
What is organisms is a gram negative bacillus, often bipolar staining and what does it cause?
|
Yersinia pestis and it causes plague
|
|
|
What three syndromes can Yersinia pestis cause depending on its route of transmission?
|
Bubonic (regional lymphadenopathy);
Septicemic; Pneumonic (would be most likely form in bioterrorism event, and resulting from aerosol exposure) |
|
|
What bioterrorism diseases use doxycycline or ciprofloxacin for post-exposure prophylaxis?
|
Plague, Anthrax, and Tuleremia
|
|
|
How is Plague diagnosed and what is the treatment?
|
Cultures of sputum and blood
|
|
|
What bioterrorism diseases use Streptomycin or gentamicin as treatment?
|
Plague, Tuleremia
|
|
|
What is the Differential Dx of Respiratory Syndrome?
|
Inhalational anthrax;
Pandemic Influenza; Plague; Tularemia; Q fever; Other atypical pneumonia (e.g. Hantavirus, SARS, Legionnaire’s Disease); Strep. pneumoniae, S. aureus, etc. |
|
|
What are the etiologies of Viral Hemorrhagic Fever (VHF) and how does it present?
|
Etiologies: Arenaviruses, Filoviruses, Bunyaviruses, Flaviviruses;
Clinical signs: high fever, headache, myalgias, bleeding; shock, Low platelet count common; also decreased WBC |
|
|
What is the differential diagnosis for Viral Hemorrhagic Fever, how is it diagnosed, & how is it treated?
|
Dx: use serologies rapid EIA; viral culture; DDx: meningococcemia, plague, Rickettsial illness (Rocky mountain fever), DIC from other causes; Treatment: ribavirin may be helpful in select cases (eg Lassa fever)
|
|
|
What features of disease would suggest bioterrorism?
|
Unusual disease or number of cases, location, or fatalities
|
|
|
What is the medical response when dealing with bioterrorism agents and disease?
|
Patient directed-isolation, treatment; Public heath directed-contact authorities at once
|
|
|
What bioterrorism diseases do NOT have person-to-person transmission?
|
Anthrax, Botulism
|
|
|
What bioterrorism disease does NOT have any available treatments?
|
Smallpox, Viral Hemorrhagic Fever (exception is Lassa fever, use Ribavirin)
|
|
|
What bioterrorism diseases have person-to-person transmission?
|
Smallpox, Pneumonic Plague, Viral Hemorrhagic Fever
|
|
|
What describes the absorption and distribution of inorganic lead (e.g. salts, oxides)?
|
30% absorbed with inhalation of dusts; 10-15% absorbed orally; 90% distributes to bone – blood levels are a poor indicator of body burden but are useful in assessing recent/ongoing exposure
|
|
|
What are the reproductive/developmental effects of lead poisoning?
|
Reduced sperm counts/motility, implicated in miscarriages/stillbirths, reduced birth weight and premature birth
|
|
|
What are the renal effects of lead poisoning?
x |
What are the renal effects of lead poisoning?
Impairs proximal tubular function, aminoaciduria, glucosuria, hyperphosphaturia |
|
|
What are the recommended actions regarding lead poisoning in children?
|
With low levels of exposure: retesting after some time period, education, and case management;
With higher levels of exposure (20-44): clinical evaluation, education, environ. investigation; With high levels of exposure: clinical evaluation/hospitalization, chelation therapy, education, environmental investigation |
|
|
What are the signs/symptoms of lead poisoning in children?
|
Lowered intelligence, diminished school performance, growth retardation, hearing loss, impaired bone growth and shortened stature, hyperactivity, sleeping problems, GI problems (stomachache)
|
|
|
How does anemia come about with lead poisoning?
|
Lead inhibits δ-ALA dehydratase, thus affecting the conversion of ALA to porphobilinogen;
It also inhibits mitochondrial ferrochelatase, decreasing conversion of protoporphyrin IX to heme– Overall these effects lead to increased ALA in plasma, excess coproporphyrinogen III in urine, and accumulation of protoporphyrin IX in erythrocytes |
|
|
What signs/symptoms are associated with lead poisoning in adults?
|
Mild (>40): CNS involvement (tiredness, somnolence), also reproductive effects and HTN;
Moderate (>80): CNS effects (more severe), GI effects (metallic taste, abd. pain), nephropathy; Severe (>100): CNS effects (severe: encephalopathy, coma, seizures), PNS effects (foot/wrist drop), GI effects, pallor due to anemia, nephropathy |
|
|
What are the two categories of metal that cause toxicity?
|
Essential metals: low intrinsic toxicity, examples incl. zinc, copper, selenium;
Nonessential metals: higher intrinsic toxicity, for ex. lead, mercury, cadmium, beryllium, arsenic |
|
|
What characterizes lead poisoning?
|
One of the most common exposures; inorganic and elemental forms are of toxicological concern
|
|
|
By what general mechanisms do metals cause toxicity?
|
Binding of sulfhydryl groups, inhibiting enzyme activity, disrupting cellular transport, precipitating proteins
|
|
|
What characterizes organic mercury toxicity?
|
With chronic toxicity, can see CNS effects (sensory defects, paresthesias, constriction of visual fields, loss of hearing/taste/smell), PNS effects (motor dysfunction, tremors, muscle incoordination), reproductive toxicity (fetus will have CNS damage at levels not toxic to mother) – the kidney is not a target organ
|
|
|
What is the biomarker for arsenic poisoning?
|
Mees lines on fingernails (transverse white lines) – also seen in other disease states
|
|
|
What characterizes arsenic poisoning?
|
People most at risk are those working the in manufacture of insecticides, weed killers, wood preservatives, and metal refining; the burning of arsenic-treated wood poses a health hazard;
The major mechanism of toxicity is to disrupt energy metabolism in the cell; Acute toxicity: affects GI, heart, neuro, hematological, liver, kidneys, will see many s/s; Chronic toxicity: skin lesions (hyperpigmentation, hyperkeratosis, allergic contact dermatitis), peripheral neuropathy, obliterative arterial disease of extremities (with groundwater contamination), anemia – antidotes incl. BAL and Succimer |
|
|
What characterizes cadmium poisoning?
|
Exists only in the inorganic form (salts/oxides), those at risk are workers in industries;
With oral ingestion 5% is absorbed, whereas with inhalation 30-60% is absorbed; Chronic toxicity leads to kidney damage (major target organ, proximal tubular damage), bone damage (osteomalacia due to disruption of calcium/phosphate homeostasis); Acute toxicity leads to lung damage (pneumonitis, emphysema) – there are no effective antidotes, chelators will cause release of cadmium in the kidneys (and thus lead to kidney damage) |
|
|
What antidotes are available for mercury poisoning?
|
Chelating agents: BAL/Succimer/DMPS for inorganic forms; elemental and organic forms are more resistant to chelation
|
|
|
What characterizes inorganic mercury toxicity?
|
Mercury salts, exposure occurs mainly by oral ingestions; Chronic toxicity can lead to GI effects (irritation of oral cavity, abd. pain, intestinal bleeding), kidney damage (major target organ, proximal tubular damage, increased excretion of amino acids, glucose, phosphate), CNS toxicity (not a major concern); Acute toxicity can lead predominantly to oral/GI toxicities (gingivitis, stomatitis, abd. pain) as well as cardiovascular collapse with severe intoxication
|
|
|
What are Minamata disease and Iraqi food exposure examples of?
|
Organic mercury poisoning from food, leading to fetal toxicity
|
|
|
What is the antidotal therapy for lead poisoning?
|
Succimer (DMSA): used for mild adult symptoms and asymptomatic children;
CaNa2EDTA, BAL: moderate/severe adult symptoms and asymptomatic/symptomatic children |
|
|
What characterizes elemental mercury toxicity?
|
Risk from inhalation of vapors (oral ingestions poses little risk due to poor absorption); Chronic toxicity is associated with CNS effects (irritability, insomnia, depression, emotional instability), PNS effects (motor dysfunction, tremors, muscle incoordination), renal toxicity (damage to proximal tubules) – acute toxicity is rare, s/s incl. GI complaints, HA, visual disturbances, and lung damage (cough, chest pain, pneumonitis at very high concentrations)
|
|
|
What characterizes mercury?
|
Toxic in elemental, organic, and inorganic forms; Exposure is primary found in a variety of occupations, combustion effluents of coal, and in food (fish/seafood)
|
|
|
What characterizes beryllium poisoning?
|
Used in many high-tech products; Acute toxicity: irritant dermatitis, eye irritation, pneumonitis, anorexia, fatigue; Chronic toxicity: berylliosis (chronic granulomatous disease in the lung, delayed hypersensitivity), allergic contact dermatitis – no antidotes available
|
|
|
What characterizes metal fume fever?
|
Seen in the welding profession, very common; Occurs within 3-12 hours of exposure to metal oxide dusts (zinc oxide, magnesium oxide, aluminum oxide, cadmium oxide, etc.);
Signs/symptoms: HA, fever, chills, muscle aches, thirst, n/v, chest soreness, fatigue, GI pain, weakness, tiredness (symptoms may last 6-24hrs, complete recovery within 24-48hrs, long-term consequences unknown) |
|
|
What metals are considered carcinogens?
|
Arsenic: skin, lungs; Chromium (hexavalent): lungs; Nickel: nose; Beryllium: lungs;
Cadmium: prostate; Lead (probable carcinogen): kidney |
|
|
What is the most common form of metal toxicity?
|
Allergic contact dermatitis: delayed hypersensitivity reaction (they are haptens); seen with beryllium, nickel, chromium, arsenic, platinum, and gold
|
|
|
What characterizes manganese poisoning?
|
Associated with Parkinson-like symptoms; seen in welding profession
|
|
|
What characterizes iron poisoning?
|
Children are at the greatest risk; Acute toxicity in children (with iron-containing supplements): irritation of GI tract/hemorrhage, liver damage (within days), renal failure, with severe exposure can see convulsions/coma/death – antidote: Deferoxamine
|
|
|
What describes the developmental period of fertilization/implantation?
|
Conception – day 17 of gestation: mitotic activity in all cells, cells are functionally equivalent in terms of totipotentiality, injury resulting in cell death can be overcome except in the case of a lethal dose (leads to spontaneous abortion/resorption of the developing organism); if recovery follows, no structural deformity in the fetus is seen
|
|
|
What aspects of the fetal-placental-maternal unit can cause fetal toxicity?
|
Direct toxicity to the fetus, damage to the placenta, indirect fetal toxicity through maternal factors (e.g. nutrition [excess vit. A], disease [DM, HTN, AIDS, thyroid dysfunction], infections [HSV, CMV, syphilis])
|
|
|
What characterizes the placental transfer of chemicals?
|
Not a very effective barrier for many chemicals; diffusion dependent on size, pKa, and lipid solubility of the chemical
|
|
|
What characterizes the susceptibility to tumor induction during gestation?
|
Seen with DES (diethylstilbestrol, used to prevent miscarriages/premature deliveries), ionizing radiation, and >50 chemicals
|
|
|
What are reasons for the CNS sensitivity during the embryonic period?
|
Proportionally high blood flow to the brain; Proportionally high lipid content relative to later in life; Poorly developed BBB
|
|
|
What describes the fetal period of development?
|
Day 56 – parturition: time of growth and development, fetus shows relative resistance to teratogens, primary malady from exposure to chemicals is reduction in cell size/number, can see growth retardation/functional deficits, developing brain is particularly prone to injury since its development (e.g. myelination) is incomplete even at birth, most common outcomes are learning disorders/behavioral problems/retardation
|
|
|
What describes the embryonic period of development?
|
18 – 55 days, organogenesis: period of extreme sensitivity to teratogenic agents (will cause structural malformations, most frequent time period involved in teratogenicity), frequently the mother will not know she is pregnant during this period; sensitivity is due to rapid cell turnover, immature biological barriers, and immature detoxicification processes (P450 system)
|
|
|
What percent of causes of developmental defect in humans can be attributed to drugs and environmental chemicals?
|
4-5% (65-70% is unknown)
|
|
|
What are the FDA pregnancy risk categories?
|
A: fetal harm appears remote;
B-D: intermediate concern; X: high risk of fetal abnormalities |
|
|
What are the final outcomes of exposure to a developmental toxicant?
|
Lethality, malformation, functional disorder, growth retardation
|
|
|
What characterizes methyl mercury?
|
Fetal exposures can result in neurological defects (mental retardation, speech disturbances) without maternal toxicity – seen in accidental mass exposures in Japan and Iraq
|
|
|
What characterizes male reproductive toxicity?
|
Chemical whose toxicity is through disruption of cell division will be more detrimental to male reproductive status than with females; Testicular damage/infertility is seen with dibromochloropropane; Decreased libido/impotence can be seen with lead, vinyl chloride, TDI; Spermatotoxicity is seen with cytotoxic drugs, dibromochloropropane, and lead
|
|
|
What characterizes retinoid embryopathy?
|
Caused by Isotretinoin use; associated with a high risk of birth defects (even with small amount for short period); birth defects incl. hydrocephaly, microcephaly, mental retardation, ear/eye abnormalities, cleft palate and other facial abnormalities, heart defects
|
|
|
What characterizes fetal effects of cocaine use?
|
Premature detachment of the placenta (vasoconstriction), increased spontaneous abortions, intrauterine growth retardation, disruption of CNS development
|
|
|
What characterizes fetal alcohol syndrome (FAS)?
|
Leading preventable cause of mental retardation and birth defects, characterized by abnormal facial features (short palpebral fissures, epicanthal folds, short/upturned nose, thin upper lips, small jaw), growth deficiencies, and CNS problems (mild/moderate mental retardation, learning disabilities, poor motor coordination, irritability during infancy, hyperactivity in childhood), urinary tract defects – native Americans have a greater incidence of FAS than other ethnic groups
|
|
|
What does fetal exposure to ethyl alcohol (i.e. ethanol) cause?
|
Fetal alcohol spectrum disorders (FASDs): umbrella term for range of effects caused by maternal ingestion of alcohol, incl. physical/mental/behavioral/learning problems with lifelong implications – most severe effects of drinking during pregnancy is fetal alcohol syndrome
|
|
|
What does fetal lead exposure cause?
|
Impaired CNS development (intelligence/cognitive development)
|
|
|
Which common drugs were initially thought to be teratogenic, but were subsequently proven to be safe?
|
Diazepam, OCs, spermicides, salicylates, Bendectin (Doxylamine plus pyridoxine)
|
|
|
What characterizes Thalidomide?
|
Fetal exposure results in amelia (congenital absence of a limb/limbs), hemimelia (congenital absence of part of an extremity), or phocomelia (defective development of arms/legs);
Critical period of exposure is between days 20-36 – Thalidomide is currently used for leprosy (Hanson’s disease) and appears promising for the treatment of multiple myeloma and SLE |
|
|
What defines neonatal and infant periods?
|
Neonate: first month of life post-partum; Infant: first year of life post-partum
|
|
|
What are the TORCH organism?
|
Organisms that can cauase transplacental infections of fetus: TOxoplasma, Rubella, CMV, Herpesvirus, and other bacterial/viral agents
|
|
|
What characterizes transplacental infections of the infant/fetus?
|
Acquired via the “hematological route” from mother to fetus: most viral/parasitic, few bacterial (Listeria, Treponema), gain access to fetal bloodstream via chorionic villi either at anytime thru delivery or at delivery via maternal-to-fetal transfusion – examples include TORCH organisms
|
|
|
What is caused by the ascending infection of candida?
|
White opacification of umbilical vessels (barber pole) and small granules – detected by GMS stain
|
|
|
What characterizes transcervical infections of the infant/fetus?
|
Acquired via the “ascending route” from cervix into uterus: most bacterial, few viral, infection can occur by either inhalation of infected amniotic fluid (resulting in congenital pneumonia) or by passage through infected cervical canal – common diseases include pneumonia, sepsis, meningitis
|
|
|
What can cervical cone biopsy to detect HPV-related cervical cancer lead to?
|
Prematurity
|
|
|
What is the 2nd most common lethal condition of neonatal period?
|
Prematurity – risk factors for prematury include preterm premature rupture of membranes, intrauterine infections, structural abnormalities of uterus/cervix/placenta, multiple gestation
|
|
|
What is a systemic way of categorizing pathological findings?
|
SNOMED: organizes pathology by T-M matrix, includes comprehensive list of systems for patient history and physical diagnosis, organizes reprint files
|
|
|
What are various perinatal pathological conditions?
|
M1: trauma: obstructed labor, fetal malpresentation
M2: congenital: 1% neural tube defects (folate deficiency), cretinism (maternal hypothyroidism), congenital syphilis M4: infections: rotavirus, syphilis, HIV, Group B strep, tetanus (shows up in infant in 7-10 days, due to lack of maternal immunity) |
|
|
What is the definition of live birth in the US?
|
All births that show any sign of life, regardless of prematurity (many countries report this as stillbirth)
|
|
|
What is placental previa?
|
Placenta attached to uterine wall close to or covering the cervix – may lead to infant death due to hypoxia during delivery
|
|
|
What is a complication of neonatal RDS?
|
Hemorrhage in germinal matrix with secondary hemorrhage into brain ventricles because blood vessels are susceptible to hypoxic damage in subependymal germinal matrix
|
|
|
What is the treatment for neonatal RDS?
|
Surfactant replacement, oxygen administered via ventilation
|
|
|
What is neonatal respiratory distress syndrome (RDS)?
|
Aka “hyaline membrane disease”, main cause is prematurity and low gestational age, is due to deficiency of pulmonary surfactant (normally decrease surface tension, production of surfactant can be stimulated by cortisol) (!) (many points from powerpoint omitted per instructor’s advice to “not worry” about them)
|
|
|
What is the mortality rate based on APGAR scores?
|
Score 0-1: 50% mortality; Score > 7: 0% mortality
Score at 1 minute assesses the need for resuscitation, score at 5 minute determines long-term prognosis of newborn |
|
|
What is the APGAR scoring system?
|
Measure of physiological condition and responsiveness of newborn: scores range from 0 to 2 for the following factors: HR, respiration, muscle tone, color, response to catheter in nostril – maximum score is 10 – scores are measured at 1 minutes and 5 minutes
|
|
|
What is twin-twin transfusion syndrome?
|
Disproportionate distribution of blood from one fetus to another when two or more fetuses share a chorion and hence a single placenta, but have separate amniotic sacs
|
|
|
What can occur with Listeria infection of pregnant mothers?
|
Can infect placenta via blood, causing abscess and massive gram (+) growth --> termination
|
|
|
What is placental abruption?
|
Premature detachment of placenta from the uterine wall
|
|
|
What conditions can cause fetal growth restriction?
|
Uteroplacental deficiency: vascular anomalies, placental abruption/previa/thrombosis/infarct or infection
|
|
|
What is necrotizing enterocolitis?
|
Ischemic colitis due to prematurity - presents with blood stools, abdominal distention, and/or circulatory collapse
|
|
|
In what conditions is Rhogam use recommended?
|
All Rh(-) women who want to become pregnant: timed at 28 weeks and delivery – rhogam is safe, single exposure may result in natural sensitization, sensitization may be life-long, after natural sensitization to Rh-antigen Rhogam is not effective (!)
|
|
|
What characterizes Rh disease of the newborn?
|
Fetus Rh(+) blood can be reacted against by maternal defense system in a mother with Rh(-) blood type who has antibodies against Rh-antigen (due to exposure of mother’s defense system to Rh(+) blood from current or previous pregnancies, etc)
|
|
|
What are findings in SIDS autopsy cases?
|
Subtle findings, mostly petechial hemorrhages (e.g. found in viscera including thymus, lungs)
|
|
|
What has occurred to the rate of SIDS over the years?
|
Declined – improvement is mainly associated with having babies sleep on their back instead of in a prone position (lying face downward)
|
|
|
Mandatory child abuse and neglect laws that require that which certain professionals and institutes are report suspected abuse to child protective services?
|
Health care providers, Teachers, School staff, Social workers, Police, Foster care providers, Daycare providers
|
|
|
What is the consequences of failure to immediately report child abuse?
|
Misdemeanor in most states, and physician can be liable for civil damages
|
|
|
What age group of children had the highest rate of victimization?
|
Birth to 3 years old
|
|
|
What is the race has the highest incidence of child abuse?
|
White (49.7%); ¼ (23.1%) were African American; and 17.4% were Hispanic (it is currently increasing in Hispanics)
|
|
|
What kind of child abuse is the most cause of death?
|
42.2% of the fatalities were attributed to neglect (76.6% of the fatalities were younger than 4 years)
|
|
|
Who is the most common perpetrators of child abuse?
|
Parents (79.4%), Relatives (6.8%), <10.1% of perpetrators were unrelated (foster parents, daycare providers and legal guardians); 57.8% of perpetrators were female
|
|
|
What are the different types of abuse?
|
Physical, Nutritional deprivation, Sexual abuse, Intentional drugging or poisoning, Neglect of medical care or safety, Emotional abuse
|
|
|
What classifies death precipitated by injury to the mother?
|
Feticide (Death can be result of premature birth)
|
|
|
What is classification of death within the first 30 days and death within 1 month to 1 year?
|
Neonaticide; Infanticide
|
|
|
In neonaticide who is most likely the responsible party if death occurs within the 1st 24, and if death occurs > 24 hours, and what is the usual cause of death?
|
First 24 hours almost always the mother; >24 hours father slightly more likely to be guilty party; COD: Asphyxia
|
|
|
Minor scalp hemorrhage, Small cephalohematoma with no evidence of skull fractures or significant intracranial injuries is classified as what?
|
Neonaticide: Delivery Injuries
|
|
|
What is the most common type of child abuse?
|
70% physical abuse, 25% sexual abuse, 5% underfeeding or neglect
|
|
|
What types of face trauma are indicative of child abuse?
|
Frenulum tears, Dislodged teeth
|
|
|
Why is full body x-rays done on all infant and pediatric death?
|
Evidence present in 1/3 of abused children, Epiphyseal-metaphyseal fractures of long bones of the arms and legs and rib fractures, Extremities 77%; Skull 34%; Rib cage 19%
|
|
|
What type extremity fracture is most often associated with child abuse?
|
Spiral Fracture
|
|
|
How does death from child abuse usually result?
|
Die as a result of head trauma: Subdural hemorrhage, Subarachnoid hemorrhage, +/- skull fractures, Brain injury; followed by abdominal injury
|
|
|
What are the signs of Elder Neglect?
|
Contractures, Malnutrition, Dehydration, Decubitus ulcers
|
|
|
What are Risk Factors for Elder Abuse?
|
Shared living situation, Dementia, Social isolation, Pathologic characteristics of perpetrators
|
|
|
What are the different stages of Decubitus ulcers and what stages are considered abuse?
|
Stage 1: reddening of the skin;
Stage 2: ulceration of the skin but it does not go past the subcutaneous tissue (needs to be clean and dry); Stage 3: through the skin & fascia, sign of abuse, must be reported Stage 4: to the bone, sign of abuse, must be reported |
|
|
What are some examples of chemicals that are poorly or not adsorbed by charcoal?
|
Alkali, DDT, organic mercury, water-insoluble compounds, etc.
|
|
|
What characterizes the use of activated charcoal?
|
Place into stomach to adsorb poison; has been reported that charcoal not only removes unabsorbed poison but also increases the clearance of systemically absorbed poison; charcoal can also interrupt the entero-hepatic cycle and increase the excretion of the poison
What characterizes the use of activated charcoal? Place into stomach to adsorb poison; has been reported that charcoal not only removes unabsorbed poison but also increases the clearance of systemically absorbed poison; charcoal can also interrupt the entero-hepatic cycle and increase the excretion of the poison |
|
|
What factors determine the effectiveness of gastric lavage?
|
Physical characteristics of the toxic agents (e.g. plants, tables, capsules, liquids), rate of absorption of toxic agents, diameter of lavage tube, volume/rate of installation of lavage solution
|
|
|
What are complications/hazards of gastric lavage?
|
Aspiration pneumonia secondary to emesis with unprotected airway, laryngospasm with cyanosis
|
|
|
What are contraindications of gastric lavage?
|
Corrosives, petroleum distillates, seizures
|
|
|
What are some reasons for the pediatric population not to take Ipecac?
|
It sometimes causes prolonged vomiting and lethargy similar to drowsiness that might be caused by an overdose of sedative drugs (symptoms may complicate diagnosis and treatment); It may also not totally empty the stomach of poison or may linger and cause the child to vomit up other antidotes; some studies have found that Ipecac did not improve outcomes
|
|
|
What are contraindications for emetic drugs?
|
When the poison ingested is a convulsant, hydrocarbon, or corrosive acid/alkali;
When the patient is unconscious/comatose, does not have a gag reflex, has severe CV disease/emphysema/extremely weakened blood vessels, is under 6 months of age |
|
|
What characterizes Apomorphine?
|
More reliable than Ipecac (in professional setting); Stimulates CTZ; does not store well (made fresh); it is a narcotic drug that causes emesis in 3-5min.; never to be used unless a narcotic antagonist (e.g. naloxone) is available to reverse the effects after emesis; should be followed with large amounts of fluid – be careful of repeating since it has mild CNS depressant effects that may act synergistically with depressants responsible for the intoxicant being treated
|
|
|
In what cases is hemodialysis effective with toxin ingestions?
|
Toxic manifestations are related to the concentration of the poison, poison is distributed in the vascular component, poison is diffusible, poison is not tightly bound to tissue/plasma proteins
|
|
|
Why are nitrites used cyanide poisoning?
|
They convert some of the hemoglobin to methemoglobin, which preferentially binds cyanide rather than having it bind cytochrome oxidase (thus negating some of its toxicity)
|
|
|
What is the antidote dimercaprol (BAL) used for?
|
Arsenic poisoning (as well as mercury poisoning)
|
|
|
In what situations are cathartics not recommended?
|
Following caustic ingestion (chemically exerting an effect resembling a burn); Bowel sounds are absent; History of recent bowel surgery; Caution with Na-containing cathartics (HTN, CHF) and Mg-containing cathartics (renal disease)
|
|
|
What characterizes the use of cathartics?
|
Used following activated charcoal to aid in evacuation of the bowels; can be given together with activated charcoal; caution with potential fluid loss and electrolyte imbalance –
examples incl. magnesium sulfate/citrate, sodium sulfate, sorbitol |
|
|
What are common substances removed by hemoperfusion?
|
Barbiturates, non-barbiturate sedatives (e.g. glutethimide, diazepam), analgesics (aspirin, methyl salicylate, acetaminophen), antidepressants, alcohols, CV drugs (not digoxin since it is tightly bound to plasma proteins), amanita phalloides, carbon tetrachloride, OPs, methotrexate
|
|
|
What are indications for hemoperfusion?
|
Deep coma or hyperactivity (caused by dialyzable drug which cannot be treated with conservative means), hypotension (threatening renal/hepatic function which cannot be corrected by adjusting circulating volume), marked hyperosmolality (which is not due to easily corrected fluid problems), severe acid/base disturbances (unresponsive to therapy), severe electrolyte disturbance (unresponsive to therapy)
|
|
|
What can be used to alkalinize the urine?
|
IV NaHCO3/Na-lactate/CA inhibitors (e.g. acetazolamide) – helpful for excretion of phenobarbital and aspirin
|
|
|
Which substances are not affected by forced diuresis?
|
Acetaminophen, glutethimide, TCAs
|
|
|
What can be used to acidify the urine?
|
IV NH4Cl/ascorbic acid – helpful for the excretion of amphetamines, quinidine, and strychnine
|
|
|
What is used to treat digitalis poisoning?
|
A steroid-binding resin (since it is tightly protein-bound)
|
|
|
What is the use of potassium salts and BB in digitalis poisoning?
|
Potassium salts and BB restore normal function by repairing or by-passing the effect of digitalis – also goes for methylene blue/agents that produce methemoglobinemia (!), guanidine/botulinum toxin, folinic acid/methotrexate, thymidine/5-FU, purines/6-MP
|
|
|
What is the use of atropine in cholinesterase inhibitor poisoning?
|
Atropine blocks receptors responsible for the toxic effects caused by the cholinesterase inhibitor
|
|
|
What is the use of oxygen in carbon monoxide poisoning?
|
Oxygen competes with carbon monoxide for essential receptors – same goes for neostigmine/curare, vitamin K/coumarins, naloxone/morphine
|
|
|
What is the use of chloride in bromide poisoning?
|
Chloride specifically accelerates excretion of bromide – same goes for calcium salts/strontium
|
|
|
What is the use of cysteine in selenocystathione poisoning?
|
Cysteine blocks metabolic formation of poison from a less toxic precursor – also goes for ethanol/methanol
|
|
|
What is the use of thiosulfate in cyanide poisoning?
|
Thiosulfate accelerates metabolic conversion of cyanide to a non-toxic product
|
|
|
What characterizes the impact of injury from conception to 17 days of gestation?
|
All-or-none response: either spontaneous abortion or resoprtion of developing organism from damage, or no structural deformity if totipotential cells recover
|
|
|
What characterizes the placental transfer of material?
|
Placenta is not a good barrier – small size, lipid-soluble, uncharged compounds pass more easily by passive diffusion
|
|
|
What are examples of compounds that increase susceptibility to tumor induction during gestation?
|
Diethylstilbestrol (DES): linked to vaginal cancer in children; ionizing radiation
|
|
|
What is the reason behind CNS sensitivity in the embryonic and fetal period?
|
High blood flow to brain, high lipid content of brain relative to later in life, poor BBB (!)
|
|
|
Which organ is especially prone to developmental toxicity during the fetal period (day 56 to birth)?
|
Brain (e.g. demyelination) because its development is incomplete even at birth – most common outcomes are learning disorders, behavioral problems, mental retardation
|
|
|
What toxicities are most seen in the fetal period (day 56 to birth)?
|
Growth retardation and functional deficits (e.g. CNS injury, retarded development) since growth in size is the primary process in this stage
|
|
|
What developmental period is associated with the highest frequence of structural defects?
|
Days 18-55 (organogenesis, embryonic period) during which pregnancy may be unknown to the mother High sensitivity in this period is due to rapid cell turnover, immature biological barriers, and immature detox processes (!)
|
|
|
What percent of developmental defects are linked to drugs and environmental chemicals?
|
5% - however, 70% of causes of developmental defects are unknown and may be due to drug and chemical
|
|
|
What are the developmental periods of fetus?
|
Conception to 17 days gestation: fertilization and implantation
Days 18-55: organogenesis, embryonic period; Day 56 to birth: fetal period |
|
|
What are possible outcomes of developmental toxicity?
|
Lethality, malformation, functional disorder, growth retardation
|
|
|
What can fetal exposure to Lead cause?
|
Impaired CNS development as measure by IQ test and cognitive development
|
|
|
Why is male reproductive system is more prone to damage from compounds that disrupt cell cycle?
|
Because male germ cell population is continually replicating
Testicular damage: Lead, dibromochloropropane, Kepone Decreased libido and impotence: Lead, vinyl chloride, TDI, Mn Spermatotoxicity: Lead, dibromochloropropane, cytotoxic drugs, carbon disulfide, radiation thermal stress |
|
|
What compounds can cause Retinoid Embryopathy?
|
Isotretinoin (even at small amounts for short periods), excess Vitamin A
|
|
|
What race has the highest risk for Fetal Alcohol Syndrome in US?
|
Native Americans
|
|
|
What characterizes Fetal Alcohol Syndrome?
|
Permanent condition: growth deficiency (prenatal, postnatal), abnormal facial features (short eye slits, short and upturned nose, thin upper lips, small jaw), CNS problems (MR, learning problems, poor motor coordination, irritable infant, hyperactive child), urinary tract defects
|
|
|
What can fetal exposure to Ethyl Alcohol cause?
|
Fetal Alcohol Spectrum Disorders (FASDs), which include Fetal Alcohol Syndrome: leading known preventable cause of MR
|
|
|
What can fetal exposure to Cocaine cause?
|
Premature detachment of placenta (due to vasoconstriction), risk for spontaneous abortion, intrauterine growth retardation, disruption of CNS development
|
|
|
What changes can lead to developmental problems?
|
Direct toxicity to fetus, damage to placenta, indirect fetal toxicity thru maternal factors (nutrition: excess vitamin A; disease: diabetes, HTN, AIDS, hypo/hyper-thyroidism; infections: herpes, CMV, syphilis)
|
|
|
What can fetal exposure to Methyl Mercury cause?
|
Neurological defects (MR, speech problems) without maternal toxicity
|
|
|
What can fetal exposure to Thalidomide cause?
|
Amelia, hemimelia, phocamelia in upper and lower limbs – critical period of exposure is days 20 to 36
|
|
|
What should be addressed in a clinical history regarding acute poisoning?
|
Who: age, weight, gender, relationship to others; What: name, dose, amount, co-ingestants;
When: time, date of ingestion; Where: route of administration, location of ingestion (e.g. at home); Why: accidental, intentional, associated details |
|
|
What characterizes Ipecac?
|
Emetic drug, must be taken with large amounts of fluid to induce emesis, takes 15-30 min.;
can be used as a home remedy; Active compounds incl. Cephaline and Emetine (active alkaloids); Produce GI irritation; Stimulated vomiting center in the medulla; Cardiotoxic if no vomited, CNS depression at high doses – not to be confused with fluid extract of Ipecac (14x more potent); not to be mixed with charcoal because it is adsorbed by charcoal and thus inactivated |
|
|
What are some important aspects of absorption following drug poisoning?
|
With the exception of aspirin and some anticholinergics, little drug remains in the gut after 4 hrs; however, delayed gastric emptying associated with pylorospasm/shock can cause retention of the drug for longer than 4 hrs (e.g. with ingestion of salicylate)
|
|
|
What is the first step in management if drug poisoning appears likely?
|
Prevent further absorption of the unabsorbed drug in the gut lumen: emesis/gastric lavage followed by activated charcoal, castor oil for highly lipid soluble drugs, efficacy of procedures depends on time interval between ingestion and removal attempt, as well as absorption of the drug
|
|
|
What should be considered regarding the differential diagnosis of a comatose patient?
|
AEIO/TIPS – Alcohol; Encephalopathy: epilepsy, encephalitis, hypertensive encephalopathy;
Insulin: hyper-/hypoglycemia; Opiates: and other CNS active drugs in high doses; Trauma: to head; Infection: meningitis, bacteremia, pneumonia; Psychiatric: hysteria, catatonia; Syncope: vasovagal attack |
|
|
What should be given to every comatose patient in whom the initial diagnosis is not evident?
|
IV hypertonic glucose (because of the real and treatable possibility of hypoglycemia) – also, save urine and vomitus for possible toxicologic assays, if needed
|
|
|
What are the initial steps in coma care during an emergency?
|
ABCDs – Airway: blocked, make patent; Breathing: chest wall/air moving, artificial respiration;
Circulation: need good gas exchange first, effective pulse beat, reasonable rhythm, no blood loss; Definitive diagnosis/treatment: need good circulation first, get IV line, draw blood for analysis, start IV drip, determine hematocrit/acetone/glucose immediately, and serum urea nitrogen/Na/Ca |
|
|
Which toxic substances are most commonly involved in child poisonings?
|
Commonly: cough/cold/pain relievers, antihistamines, antidepressants, sedatives, CV drugs;
Spring time: pesticides, herbicides, fertilizers – 2yo child represents peak age for poisoning risk |
|
|
What toxic substances are most commonly associated with death after ingestion?
|
Antidepressants, analgesics, stimulant/street drugs, CV drugs, alcohols/glycols, chemicals, etc.
|
|
|
What are the most common substances involved in human poisonings (all ages)?
|
Cleaning substances, analgesics, cosmetics, cough/cold medicines, plants, bites (insect/snake), etc.
|
|
|
What are indications for gastric lavage?
|
Risk/benefit ratio (risk of aspiration with unconscious patient is reduced by using cuffed endotracheal tube), semiconscious, unconscious child/adult, loss of gag reflex, Ipecac-induced emesis is ineffective or contraindicated, conscious patient ingesting large quantity of highly toxic substance – continue until all particulate material is removed (clear fluid)
|
|
|
What are mechanisms by which the metabolism and elimination of toxins can be hastened?
|
Metabolism to inactive compounds by the liver microsomes, binding of active drug to protein or lipid-binding sites, excretion of active drug by the kidney, maintenance of proper oxygenation/ temperature/perfusion of vital organs, maintenance of brisk urinary output, acidification/ alkalinization of urine (in amphetamine and phenobarbital intoxication, respectively)
|

