![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
207 Cards in this Set
- Front
- Back
|
Objectives of first anemia ppt: |
Describe the characteristics and significance of the following classes of anemias and identify the most common examples of each class: *Megaloblastic anemias *Hemolytic anemias - both inherited and acquired
Describe the diagnostic roles of folic acid assay, Vitamin B12 assay, Reticulocyte count, ESR, and Hemoglobin electrophoreisis |
|
|
Megaloblastic anemias have two major divisions. What are they? |
Vitamin B12 Deficiency and Folic Acid Deficiency |
|
|
What does Megaloblastic refer to? |
Abnormal bone marrow red cell precursors that show altered DNA synthesis; very large blood cells because DNA synthesis is inhibited and the cell continues growing without division. |
|
|
What are the characteristics of red cells in megaloblastic anemia? |
Abnormal nuclear maturation Imbalance between nuclear and cytoplasmic maturation (n/c ratio?) This is bc the absense of B12 or Folate impairs DNA synthesis, slowing down each step of maturation. |
|
|
What are some cellular results of megaloblastic anemia? |
Large nucleus, increased cytoplasmic RNA, early hemoglobin synthesis
Many cells never undergo mitosis and breakdown in bone marrow, so serum LDH is increased |
|
|
What other cell types does Megaloblastic anemia affect? |
Myelogenous white cells, megakaryocytes (producing leukopenia and neutrophilic hypersegmentation and thrombocytopenia) |
|
|
True or False: Megakaryocyte fragments and giant platelets can be seen on peripherial blood smears |
True |
|
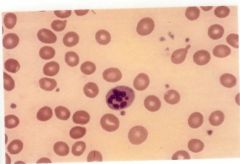
What type of Anemia is depicted in this image? |
Megaloblastic Anemia |
|
|
True or False: Neurological symptoms are rate in megaloblastic anemia |
False; they are common. They may even occur in the absence of hematological symptoms. |
|
|
How is megaloblastic anemia diagnosed? |
Serum Cobalamin (B12) or Folate is measured |
|
|
Megaloblastic Anemia caused by B12 deficiency is associated with these disorders: |
-Parasitic infection (D. latum), which causes increased cobalamin (B12) usage -Malabsorption syndromes -Nutritional deficiency -Pernicious anemia, associated with chronic atrophic gastritis (disease with auto-antibodies to intrinsic factor which is needed to absorb B12)
BASICALLY, something is stopping B12 from being absorbed, it is not consumed enough. |
|
|
Megaloblastic Anemia caused by folic acid deficiency is associated with: |
-Abnormal absorption -Increased utilization (pregnancy) -Treatment with antimetabolites that act as folic acid antagonists
BASICALLY, not absorbed enough, overused, or depleted by drugs |
|
|
Normal red cell maturation relies on two factors. What are they? |
Vitamin B12, Folate |
|
|
Where does Vitamin B12 occur? |
Naturally, in meats, eggs, and dairy. |
|
|
Where are folates found? |
Yeast, leafy vegetables, organ meats |
|
|
What is the most common megaloblastic anemia? |
Pernicious anemia; its laboratory findings are similar to other types of megaloblastic anemias and will look the same regardless of cause (B12 or folic acid deficiency) |
|
|
True or False: In megaloblastic anemia, Hemoglobin and red counts are extremely high |
False, they are extremely low. |
|
|
How is hematocrit affected by megaloblastic anemia? (MCV, MCH, MCHC) |
Hematocrit may not reflect the actual red cell decrease because the cells are so large.
MCV - as high as 130 (normal: 80-100) MCH - varies, but is usually increased (normal: 27-31) MCHC - usually normal (normal: 32-26) |
|
|
How does megaloblastic anemia affect platelets? |
Platelet counts are moderately decreased |
|
|
How are white cells affected by megaloblastic anemia? |
Leukopenia, usually neutropenia (low WBC count, particularly neutrophils that fight bacterial and fungal infections) |
|
|
What would a megaloblastic anemia smear look like? Describe the features. |
-Moderate to significant anisocytosis (blood cells are unequal in size) and poikilocytosis (abnormally shaped blood cells), with many macrocytes (large cells) and ovalocytes (oval shaped and hgb rich) -Erythroid precursors, mostly metarubicytes (nucleated RBC) -Red cell inclusions such as basophilic stippling, Howell-Jolly bodies, and Cabot Rings -Leukocytes show hypersegmentation and an increase in eosinophils -Platelets may be decreased with giant forms present -Reticulocyte count is low -Pancytopenia will occur in advanced cases (reduction in red cells, white cells, platelets) -Dimorphic red cell population w/ high RDW (red cell distribution width) |
|
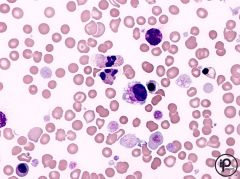
What type of anemia is this? |
Megaloblastic anemia |
|
|
What occurs in the bone marrow as a result of megaloblastic anemia? |
-Hypercellular with megaloblastic changes in the erythroid line or all lines -RBC precursors are enlarged with decreased n/c ratio -Giant metamyelocytes with large, incompletely segmented nuclei -Increased iron stores |
|
|
What are the chemistry results for megaloblastic anemia cases? Consider LDH, bilirubin, and uric acid levels. |
-Extremely high LDH -Increased unconjugated bilirubin (due to intramedullary hemolysis) -Uric acid levels are low, secondary to decreased DNA synthesis |
|
|
What tests can be performed to diagnose megaloblastic anemia? |
-Vitamin B12 assay -Folate Assay -Schilling test (to detect B12 and intrinsic factor deficiency)
[Radio-labeled B12 is given. If the urine specimen shows little radio-label, then there is a B12 deficiency. If radio-label appears in urine, a second test is given. If the urine is clear, the diagnosis is pernicious anemia. If radiolabel appears in the urine, there is a malabsorption syndrome] |
|
|
How is megaloblastic anemia treated? |
-The patient is given B12 or folate -After treatment, the reticulocyte count begins to increase in 2-3 days and it will peak at 5-8 days. -Hematocrit will begin to increase in 1 week and will normalize in 4-8 weeks. |
|
|
What is hemolytic anemia characterized by? |
-An increase in red cell destruction, initiated primarily by cells being trapped in the liver or spleen. This produces a decrease in the normal erythrocyte lifespan. |
|
|
What is the bone marrow response to increased red cell destruction? |
Increased bone marrow activity will occur to compensate for the decrease of red cells. If the bone marrow fails to keep up with the red cell destruction, anemia develops. |
|
|
Hemolysis of the red cell involves the alteration of the membrane. The causes for this alteration can be divided into two categories. What are they? |
-Intrinsic hemolytic anemia (inherited) -Extrinsic hemolytic anemia (outside factors affected the cell) |
|
|
Describe the destruction sites of red cells. |
Intravascular (within circulating blood) or extravascular (outside circulating blood) |
|
|
List some causes of inherited hemolytic anemia. |
-Membrane defects (Heriditary spherocytosis [spherical blood cells], elliptocytosis [ellipse shaped blood cells], stomatocytosis [blood cells are leaky], Rh null disease)
-Enzyme defects (G6PD deficiency [favism, normally protects red blood cells), pyruvate kinase deficiency, methemoglobin reductasse deficiency [ferric rather than ferrous hbg; difficulty releasing oxygen to tissues])
BASICALLY, can affect membrane structure, enzymes, or hemoglobin molecule |
|
|
Normal red cell membranes allow the cell to be flexible, resilient, and undergo numerous passages through the spleen. They normally survive for 120 days. When the cell membrane becomes instable and inflexible, what happens? |
The spleen must remove the cell. The rate of hemolysis is increased and will exceed the bone marrow's ability to replace the cells. Anemia will occur. |
|
|
The ability of a red cell to deform and return to normal is determined by: |
-Membrane flexibility (structure and functional integrity of membrane skeleton) -Cytoplasmic viscosity (determined by hbg) -Cell surface area to volume ratio |
|
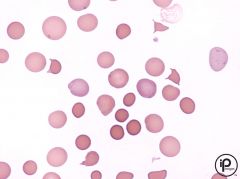
-In this disorder, cases can range from almost normal trait carriers to severe anemics. -A membrane protein defect can cause a loss of portions of RBC membrane. -Hemolysis is extravascular, occurring only in the spleen. -Decreased surface area to volume ratio causes the cell to deform (reduced flexibility) -Abnormal permeability to Na+ ions, allowing lots of sodium in (10x) |
Heriditary spherocytosis |
|
|
What is the MCH, MCV, and MCHC in heriditary spherocytosis patients? |
-MCV is normal to slightly decreased -MCH is normal -MCHC is greater than 36% |
|
|
How is heriditary spherocytosis diagnosed? |
Osmotic fragility test, splenectomy can correct the patient's anemia and hemolysis in severe cases but the basic membrane defect will remain and spherocytes may still be present. |
|
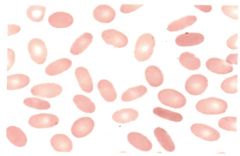
-Caused by a defect in the membrane skeleton -Most prominent peripherial blood smear finding is an increase in malformed cells (over 25% of population) -Membrane may fragment under circulation stress and produce RBC fragments |
Hereditary elliptocytosis |
|
|
True or False: Cells have a normal lifespan when affected with hereditary elliptocytosis |
True |
|
|
True or False: About 60% of patients with hereditary elliptocytosis will have significant chronic hemolytic anemia |
False, only about 10% |
|
|
How is hereditary elliptocytosis treated in symptomatic patients, and what are the results? |
Splenectomy; hemolysis is prevented (most patients show little to no hemolysis in the first place) but elliptocytes will remain |
|
![-This disorder can be seen in the genetic defect, thalassemia [abnormal hemoglobin production], lead poisoning, and alcoholic cirrhosis.
-The blood cells contain increased sodium and decreased potassium
-The cells are overhydrated
-T...](https://images.cram.com/images/upload-flashcards/45/25/35/8452535_m.jpg)
-This disorder can be seen in the genetic defect, thalassemia [abnormal hemoglobin production], lead poisoning, and alcoholic cirrhosis. -The blood cells contain increased sodium and decreased potassium -The cells are overhydrated -The anemia is usually mild -Bilirubin is increased and reticulocytosis is moderate -The smear shows 10-50% abnormal cells characteristic of this anemia type |
Hereditary stomatocytosis |
|
|
What is the MCHC and MCV like in hereditary stomatocytosis? |
MCHC is decreased, MCV is increased |
|
|
-This is a rare hereditary disorder associated with stomatocytosis, spherocytosis, and the deletion of all Rh-Hr determinants on RBC. -The result is mild anemia (Hbg 11-13 g/dl) -Reticulocytes are moderately increased. |
Rh_null Disease |
|
|
Red cells in Rh_null disease are of these types. |
Spheroidal or stomatocytic |
|
|
The levels of this type of hemoglobin are elevated in Rh null Disease. |
Hgb F |
|
|
These are types of enzyme defects causing Hemolytic Anemia. |
G6PD, Pyruvate Kinase, Methemoglobin Reductase |
|
|
-Development of this type of hemolytic anemia is related to drug intake and oxidant stress when patients have this type of defect. -Red cells cannot compensate for unstable enzymes bc they lack the organelles -An excess of oxidized glutathione accumulates and forms insoluble complexes with hemoglobin -> Heinz Bodies form |
G6PD Deficiency |
|
|
These substances can cause anemia in G6PD deficient patients. |
Primaquine, Chloramphenicol, Quinine, Quinidine, and Fava Beans |
|
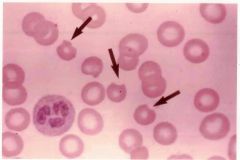
Describe the lab findings in G6PD Deficient patients. Consider G6PD levels, autohemolysis tests, and bodies that would be present on a stain. Also note what is indicated in the image that may be a clue to diagnosis. |
-G6PD levels reduced -Positive autohemolysis test -Presence of Heinz bodies on a supravital stain (NOT visible on Wright's stain) -Bite bodies |
|
|
-This enzyme deficiency results in the deficiency in generating ATP. -Results in water loss and cell shrinkage, shape distortion, and rigidity in membrane -Premature red cell destruction in the spleen and liver resulting in hematolytic anemia -Peripherial smear will look normochromic, normocytic with varying degrees of polychromatophilia [ The extra staining is due to too many immature red blood cells (RBCs) called reticulocytes. These cells have a blue colored center.] -Blood smear looks normal -Infants in the US are screened for this at birth |
Pyruvate Kinase (PKU) |
|
|
-In this enzyme deficiency, about 1% of circulating hemoglobin in normal individuals is this type (ferric iron instead of ferrous iron) -The predominant clinical effect is cyanosis because oxygen cannot effectively be carried to tissues |
Methemoglobin Reductase Deficiency |
|
|
What are some causes of acquired hemolytic anemia? |
-Chemicals, drugs, venoms -Infectious microorganisms -Immune mechanisms -Physical agents -Trauma *See Slide 55, Anemias powerpoint* |
|
|
True or False: Intravascular hemolysis can result from exposure to environmental agents and persists even after the condition is no longer present. |
False, conditions usually cease once the threat is gone. |
|
|
Quinine sensitivity can cause this. |
Hemolytic Uremic Syndrome |
|
|
What are symptoms of Adult Hemolytic Uremic Syndrome? |
Microangiopathic hemolytic anemia, thrombocytopenia, renal failure, neutropenia, low grade DIC [Disseminated intravascular coagulation] |
|
|
Drugs: What is the preferred target for drug-induced antibodies? |
Platelets, but white cells, red cells, and other tissues are sometimes affected |
|
|
Chemicals: What effect can chemicals (lead from gasoline, paint, and industrial products) have on blood production? |
Interfere with ATP production and inhibit heme synthesis |
|
|
Venoms: Venoms from some type of snakes contain this, which can produce intravascular hemolysis and initiate DIC [Disseminated intravascular coagulation] |
Hemolysins |
|
|
True or False: Infectious Microorganisms (viruses, bacteria, and protozoa) can cause the destruction of red blood cells |
True |
|
|
This infectious microorganism can rupture red cells. |
Intraerythrocytic protozoa (malaria) |
|
|
This infectious microorganism releases a hemolytic toxin, in some cases. |
Clostridium perfringens |
|
|
This infectious microorganism has emerged as a major cause of hemolyric-uremic syndrome. |
E. coli O157:H7 |
|
|
The immune hemolytic anemias are grouped according to the presence of these things. |
Auto-antibodies, iso-antibodies, and drug related antibodies. |
|
|
This is caused by an altered immune response which results in the production of antibodies against one's own red cells, resulting in hemolysis. |
Autoimmune hemolytic anemia |
|
|
This type of anemia has been associated with Mycoplasma pneumonia and infectious mononucleosis. |
Autoimmune Hemolytic Anemia |
|
|
True or False: Autoimmune Hemolytic Anemia can be caused by cold antibodies only. |
False; can be caused by warm or cold antibodies |
|
|
Usually, warm antibodies are in this class. Hemolysis occurs in this body part. |
IgG; the spleen *See chart on slide 65 on Anemias ppt* |
|
|
Cold antibodies are this class. Hemolysis usually occurs in this way. |
IgM; intravascularly |
|
|
This type of anemia usually occurs in newborns because of maternal antibodies crossing the placenta that are directed against antigens on the baby's cells. |
Isoimmune Hemolytic Anemia |
|
|
Isoimmune Hemolytic Anemia is the most common cause of this type of blood incompatibility reactions between mother and baby. |
ABO incompatibility |
|
|
This type of anemia may occur following administration of insulin, antihistamines and sulfonamides. |
Drug Induced Hemolytic Anemia |
|
|
Drug Induced Hemolytic Anemia is this class and results in this event. |
IgM; hemolysis |
|
|
Hemolysis can occur within 24 hours of this event. In addition to hemolysis, what else occurs? |
Suffering severe burns; because the red cells become fragile after heat exposure, they form fragments and microspheres. These are removed by the spleen and a rapid drop in circulating red cell volume occurs |
|
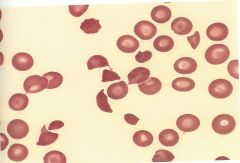
This is an example of a traumatic of microantiopathic hemolytic anemia. It's a consumptive coagulopathy that involves the deposition of microthombi in the blood cells. These deposits damage circulating red cells. |
DIC |
|
|
Traumatic injury from repeated impact of the capillary bed can produce red cell damage. What are some examples/causes of this? |
-March anemia (diagnosed in soldiers after intense marches) -Long distance runners -High impact sports |
|
|
How long do normal red cells last in circulation? |
120 days |
|
|
True or False: As the cell ages, membrane changes occur that cause the spleen to recognize and signal for phagocytic destruction of the cell |
True |
|
|
Most red cell destruction is [intravascular/extravascular] |
Extravascular |
|
|
What occurs when red cells are destroyed? |
-Red cell is reduced back to heme and globin -Globin is reduced into amino acids and recycled to make new proteins -Heme is broken into iron + CO +biliverdin -Iron is recycled into new hemoglobin -Biliverdin is converted to bilirubin and ultimately excreted in the billary system *Make a chart later; see slide 73 in first ppt* |
|
|
When the RBC destruction is increased, the formation of this exceeds the liver's ability to conjugate it. This produces elevated levels of the substance in the blood, causing jaundice. |
Unconjugated billirubin |
|
|
If intravascular hemolysis occurs, this is present in the blood. |
Free hemoglobin |
|
|
What happens to free hemoglobin in the blood? |
It is rapidly bound to the carrier protein Haptoglobin; a large complex that cannot pass through the renal glomeruli |
|
|
This is a large complex that cannot pass though the renal glomeruli. It binds to free hemoglobin. |
Haptoglobin |
|
|
If the amount of intravascular hemoglobin exceeds the amount of haptoglobin present, what may happen? |
The remaining free hemoglobin may pass through the glomeruli and be excreted in the urine |
|
|
A patient with hemolytic anemia is expected to have these symptoms, as determined by diagnostic tests: |
-Decreased hemoglobin -Decreased hematocrit -Decreased red cell count -Blood smear showing spherocytes (hallmark abnormality of hemolytic anemias) |
|
|
What is the hallmark abnormality of hemolytic anemia? |
Spherocytes |
|
|
What are some red cell abnormalities that explicitly described in the chapter? |
-Acanthrocytes -Schistocytes -Polychromasia -Target Cells -Early red cells -Increased reticulocyte count |
|
|
Clinical Chemistry Assays are affected by red cell destruction caused by hemolytic anemia. What would these tests indicate about unconjugated biirubin and serum haptoglobin? |
Unconjugated Bilirubin - rarely exceed 3-4 mg/dl
Serum Haptoglobin - Decreased levels in the presence of free hemoglobin |
|
|
This is an intravascular hemolytic disorder that is characterized by intermittent sleep-associated blood in the urine. |
Paroxysmal Nocturnal Hemoglobinuria (PNH) |
|
|
In PNH, these events occur: |
-Red cell defect that renders the red cells to be very sensitive to the complement -25% of cases will evolve into or from aplastic anemia -10% will develop acute myelogenous leukemia |
|
|
Some clinical manifestations of Paroxysmal Nocturnal Hemoglobinuria (PNH) may include: |
-Chronic Hemolysis -Thrombosis -Recurrent Infections -Tendency toward bone marrow aplasia |
|
|
Describe laboratory findings regarding Paroxysmal Nocturnal Hemoglobinuria (PNH) |
-Severe anemia HGB - less than 6 g/dl -Blood smears show hypochromic microcytic cells (result of iron deficiency) -Sucrose hemolysis and Hamm tests show increased hemolysis -Hemosiderinuria (excretion of iron from red cell destruction; a classic sign of chronic intravascular hemolysis) |
|
|
What is a classic sign of chronic intravascular hemolysis? |
Hemosiderinuria (Excretion of iron from red cell destruction) [Brown urine] |
|
|
What are the treatment options for Paroxysmal Nocturnal Hemoglobinuria? |
Transfusion therapy, antibiotics, anticoagulants |
|
|
Objectives of second anemia ppt: |
-Describe the clinical signs and symptoms of anemia and common lab tests used to assess anemia -Describe the characteristics and significance of the following classes of anemia and idenfity the most common examples *Anemia of blood loss *Aplastic anemia *Hypochromic anemias *Anemias related to iron metabolism |
|
|
Anemia is considered to be present under which condition? |
When the hemoglobin concentration is below the normal reference range. |
|
|
What are the three causes of anemia? |
-Blood Loss -Impaired red cell production -Accelerated destruction of red cells (Hemolysis) |
|
|
True or False: Anemia often causes many disorders. |
False, anemia is more often a sign or symptom of an underlying disorder |
|
|
What are the signs and symptoms of anemia a result of? |
Diminished oxygen delivery to tissues, decreased hemoglobin, lowered blood volume |
|
|
True or False: Anemics will always show symptoms |
False, if anemia develops slowly, a patient may not show any signs or symptoms until Hgb reaches concentrations as low as 6 g/dl
Note: Normal levels: * Adult males: 14 to 18 gm/dL* Adult women: 12 to 16 gm/dL |
|
|
Usual signs and symptoms of anemia include: |
-Fatigue -Dizziness -Weakness -Headache -Pallor -Low blood pressure -Slight fever -Edema |
|
|
How is anemia physiologically defined? |
A condition in which the circulating blood lacks the ability to adequately oxygenate the body tissues |
|
|
Three major laboratory manifestations of anemia are: |
-Decreased hemoglobin concentration -Decreased packed cell volume (hematocrit) -Decreased red cell count |
|
|
Addition to hemoglobin tests, hematocrit tests, and a packed red cell count, these tests are also performed when diagnosing anemia: |
-Red cell indices (MCV, MCHC, MCH) -Red cell histogram -Red cell distribution (RDW) |
|
|
Erythrocyte changes are reported in this way: |
Semiquantitatively; (Moderate, Marked, Mild) (1+, 2+, 3+, 4+) |
|
|
There are two types of anemias of blood loss. What are they? |
Anemia from acute blood loss Anemia from chronic blood loss |
|
|
This type of anemia is associated with traumatic conditions such as accidents or severe injury. It does not occur immediately. |
Acute blood loss anemia |
|
|
Severe hemmorage of more than ____% of circulating blood volume reduces the total blood volume and produces these problems. |
20; shock and related cardiovascular problems |
|
|
Severe acute bleeding can be fatal because this may occur. How can it be remedied? |
Collapse of the circulatory system; immediate expansion of blood volume is required |
|
|
How does the body respond to acute blood loss? |
It will adjust to the situation by expanding the circulatory volume, which produces the subsequent anemia. Fluid from extravascular spaces enter the blood circulation and dilutes the remaining cells. |
|
|
True or False: Lab findings for patients with acute and chronic bleeding are very similar, sometimes identical. |
False; they can vary. |
|
|
Describe the difference in lab findings between acute and chronic blood loss anemia. |
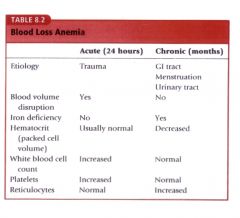
|
|
|
What is the first hematologic change that can be seen in an patient suffering from acute blood loss anemia? |
A fall in platelet count; this is due to platelets being used by the body in an effort to stop the bleeding
NOTE: The platelet levels may rise as the bone marrow responds (within an hour of blood loss) |
|
|
What is the second change seen in acute blood loss anemic patients? |
An increase in the WBC count and a "shift to the left" (leukocytosis)
NOTE: Hemoglobin and Hematocrit do not decrease immediately, but will decrease as the tissue fluids move into blood circulation (within 48-72 hours) |
|
|
What will an acute blood loss anemic patient's smear look like after 24 hours? MCV? MCH? MCHC? |
Normochromic and normocytic blood cells, assuming the patient was healthy beforehand. MCV, MCH, and MCHC will also appear normal. |
|
|
How many days does it take reticulocytes to enter circulation after acute blood loss has occurred? |
3-5 days |
|
|
At what day is the maximum number of reticulocytes present in circulation following acute blood loss? |
10 days |
|
|
How many days will it take for WBC count to return to normal after acute blood loss? |
2-4 days |
|
|
How long will it take for the "shift to the left" to no longer be present following acute blood loss? |
2 weeks |
|
|
This type of anemia is caused as a result of GI tract disorders and/or in women with heavy menstruation or UT abnormalities. The bleeding extends over a period of time, usually months, and occurs in small amounts. |
Chronic Blood Loss Anemia |
|
|
How is blood volume affected by chronic blood loss? How is the rate of red cell replenishment affected? How is the reticulocyte level affected? |
Blood volume is unaffected because loss is slow; red cells are replenished at a slower rate than acute loss; reticulocytes may be normal or slightly increased |
|
|
What would the red cells look like in a patient with chronic blood loss anemia? |
Hypochromic and microcytic |
|
|
CASE STUDY 1: A 38 year old white woman was treated in the emergency department for severe lacerations and possible abdominal injuries sustained in an automobile accident. She was admitted to the hospital for observation and further evaluation. On admission, a complete blood count, urinalysis, and radiograph series were ordered.
LAB DATA: Hemoglobin: 10.5 g/dl Hct: 0.34 L/L RBC: 3.8 * 10^12/L WBC: 12 * 10^9/L MCV: 89.6 fL MCH: 27.6 pg MCHC: 31%
The Peripherial Blood Smear showed essentially normal RBC morphology and platelet distribution. 48 hours later, the tests were re-ordered. These were the results: Hct: 0.26 L/L RBC: 2.9 * 10^12/L WBC: 15.5 * 10^9/L
The RBC indices were all within normal ranges. Peripherial blood smears indicated that morphology was mostly normal aside from some polychromatophillia. The distribution of platelets was 0.60*10^12/L. The laparotomy determined that the patient had liver and spleen injuries.
1. Why was the patient's hemoglobin and hematocrit normal on admission, but decreased after 48 hours? 2. What is the significance of the patient's increased leukocyte (WBC) and thrombocyte (platelet) count? 3. What is the reason for the polychromatophilia noted on the 48 hour film? |
1.The body adjusts to severe hemorrhaging by expanding the circulating volume at the expense of the extravascular fluid. The volume adjustment produces a delayed anemia. As the extravascular fluid enters the blood stream, it dilutes the RBCs. The hemoglobin and hematocrit become decreased after 48 hours and 72 hours in some cases.
2. In acute blood loss, the platelets and circulating granulocytes increase within a few hours. Immature WBCs may also be seen. Increased leukocytes and platelets are a normal body's response to stress. The body continually strives to maintain homeostatis.
3. Acute blood loss immediately begins to stimulate a healthy, normal bone marrow. Reticulocytosis becomes apparent within 24 hours and peaks at 7-10 days after severe blod loss. When erythrocyte restoration is completed, reticulocytosis ceases. Reticulocytes are seen as polychromasia or polychromatophilic RBCs when the RBCs are stained with a Wright stain. A supervital stain such as a new methylene blue stain needs to be used to visibly demonstrate reticulocytes in erythrocytes.
DIAGNOSIS: Acute blood loss |
|
|
CASE STUDY 2: A 55 year old white male college professor has been experiencing fatigue and shortness of breath when walking over the past several months. Getting more sleep at night did not help. He reported eating a balanced diet of fruits, vegetables, meat, and dairy products. Upon physical examination, he appeared slightly pale but no other abnormalities. His primary care physician ordered a complete blood count, urinalysis, and fecal occult blood (x3) tests.
LAB FINDINGS: Hemoglobin 12.5 g/dl Hematocrit 0.32 L/L RBC 4.2 * 10^12/L WBC normal MCV 42 FL MCH 29.7 pg Urinalysis normal Fetal occult blood POSITIVE
1. What is the most likely cause of this patient's anemia 2. What type of red blood cell morphology would be expected on a peripheral blood smear? 3. What follow-up tests should be conducted? |
1. The most likely cause of this patient's anemia is chronic blood loss. The source of the bleeding could be the GI tract, in view of the fact that he had a positive test result for fecal blood.
2. Hypochromic, microcytic red blood cells would be expected on the peripheral blood smear.
3. The source of the fecal blood needs to be located. In this case, the patient had a follow-up colonoscopy that revealed a number of non-malignant, bleeding polyps. |
|
|
This is one of a group of disorders known as hypoproliferative disorders that are characterized by a decreased production of blood cells. |
Aplastic Anemia |
|
|
Other anemias in the category of hypoproliferative disorders, aside from Aplastic Anemia, are caused by deficiencies of: |
-Erythropoietin -Iron -Folic Acid -Vitamin B12 |
|
|
The major form of aplastic anemia is: |
Idiopathic (of unknown origin) |
|
|
Aside from idiopathic aplastic anemia, what are the two other forms discussed in class? |
Iatrogenic (drug related) and Constitutional (congenital or genetic predisposition to bone marrow failure) |
|
|
This type of anemia occurs after drug exposure or exposure to cytotoxic chemicals, seen in chemotherapy or radiation therapy. |
Iatrogenic Aplastic Anemia |
|
|
How do chemicals result in iatrogenic aplastic anemia? |
Chemicals can injure proliferating and quiescent hematopoietic cells which can lead to the damage of DNA and ultimately cell death. |
|
|
What are some examples of Iatrogenic agents? |
-Benzene and Benzene derivatives -Trinitrotolune -Insecticides and weed killers -Inorganic arsenic -Antimetabolites (antifolate compounds and analogues of purines and pyrimidines) -Antibiotics |
|
|
This type of anemia is characterized by total bone marrow failure with a reduction in circulating levels of red blood cells, white blood cells, and platelets. It is uncommon. |
Aplastic Anemia |
|
|
What are the two onset types of aplastic anemia? |
Acute (profound pancytopenia and rapid progresion to death) or insidious (chronic disease course) |
|
|
Signs and symptoms of Aplastic Anemia depend on the degree of deficiencies. They include: |
-Bleeding from thrombocytopenia -Infection from neutropenia -Anemia (obviously? huh.) |
|
|
True or False: Sphenomegaly and lymphadeopathy are present in patients with aplastic anemia. |
False; they are absent |
|
|
What are aplastic anemia survivors predisposed to? |
Paroxysmal nocturnal hemoglobinuria (PNH), Myelodysplasia, and acute myelogenous leukemia |
|
|
Describe the laboratory findings of Aplastic Anemia. |
-Caused by damage to the hematopoietic tissue of bone marrow that results in deficient blood cell production -Pancytopenia - all cell lines are affected ***IF ONLY ONE CELL LINE IS INVOLVED, it is usually the red cell line*** -If two or more cell lines are affected, it's referred to as severe aplastic anemia
-Red cells: normochromic and normocytic [in some cases, there may be varying degrees of anisocytosis and poikilocytosis or macrocytosis -Serum iron is increased [this is a valuable early sign of erythroid hypoplasia and reflects decreased plasma iron turnover] -Bone Marrow exam shows very few early erythroid and myeloid cells, few to none megakaryocytes |
|
|
True or False: Aplastic Anemia is a poor prognosis, with a high mortality rate. |
True |
|
|
What are some complications of aplastic anemia? |
-Infections -Bleeding -Problems from iron overload and repeated transfusions |
|
|
What are some modern treatment options for aplastic anemia that have improved its mortality rate? |
Immunosuppressant therapy and bone marrow transplant |
|
|
This is a congenital form of aplastic anemia. |
Fanconi's Anemia |
|
|
What are the clinical signs of Fanconi's Anemia? |
-Low birth weight -Skin hyperpigmentation -Short stature
Other manifestations: -Skeletal disorders -Renal malformations -Microcephaly -Hypogonadism -Mental retardation -Strabismus |
|
|
Describe the lab findings of Fanconi's Anemia |
-Progressive pancytopenia usually apparent by age 5 -Hemoglobin concentration 5-6 g/dl at diagnosis
|
|
|
What is the treatment for Fanconi's Anemia? |
Bone marrow transplant or cord blood treatment |
|
|
This is the hypoproliferation of the red cell line without a corresponding decrease in other cell lines. |
Pure red cell aplasia |
|
|
Some causes of red cell aplasia are: |
-Congenital - Diamond-Blackfan syndrome -Acquired chronic - Idiopathic, associated with thymoma and lymphoma -Acute (transient) - parvovirus, other infections, drugs, riboflavin deficiency |
|
|
This is a rare congenital form of red cell aplasia, resulting from a defective stem cell, probably the committed erythroid stem cell. It is characterized by a slowly progressive refractory anemia with no concurrent leukopenia or thrombocytopenia |
Diamond-Blackfan Syndrome |
|
|
This is the deficiency of red blood cells that does not readily yield to treatment |
Refractory anemia |
|
|
Describe the lab results for Diamond-Blackfan syndrome |
-Occurs in early infancy -Severe normochromic, slightly macrocytic anemia -Bone marrow shows reduction in erythroid precursors but normal granulocytic and megakaryocytic lines -Fetal Hgb is elevated (5-25%) -Most patients respond to steroid treatments |
|
|
This is one of the most commonly encountered deficiency-related anemias. |
Iron deficiency anemia |
|
|
What are the four conditions that can result in iron deficiency anemia? |
-Nutritional deficiency -Faulty or incomplete iron absorption -Increased demand for iron that is not met -Excessive loss of iron |
|
|
Dietary iron has two sources. What are they? |
Non-heme iron and heme iron |
|
|
What are the two forms that iron is stored? |
Ferritin and hemosiderin |
|
|
What are the clinical signs and symptoms of iron deficiency in children? |
Psychomotor and mental impairment in the first two years of life and Pica |
|
|
Describe the lab findings in iron deficient anemia. |
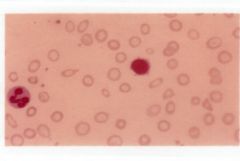
-Decreased Hbg and Hct -Red cell counts are normal initially, but will decrease as the iron deficient state continues -Decreased MCV, MCH, MCHC -Hypochromic, microcytic cells -Platelets are normal -Leukocytes are normal -Reticulocytes are decreased or normal -Bone marrow exam reveals marked decrease in sustainable iron
|
|
|
Describe the chemistry-based lab findings for iron deficiency anemia. |
-Serum iron: significantly decreased -Total iron binding capacity: increased -Percent saturation: Significantly decreased -Serum ferretin: decreased***
***Currently accepted lab test for iron deficiency diagnosis; significantly decreased levels is highly specific for iron deficiency |
|
|
True or False: Any inflammatory condition that lasts for at least two weeks can produce anemia. |
False; lasts for 1-2 months |
|
|
Anemia of inflammation can result from: |
-Decreased release of iron from the RE system -> decreased iron supply to bone marrow -Inappropriately decreased erythropoietin production -Suppression of erythropoiesis by cytokines (from the inflammatory response) |
|
|
Anemia associated with malignancy can be caused by: |
-Decreased red cell production because of bone marrow infiltrated by a tumor -Release of cytokines by microphages in response to a tumor -Increased red cell destruction due to hemolytic anemia -Acute and chronic blood loss -Toxic effects of treatment |
|
|
Describe the lab results for anemia of inflammation. |
-Mild hypoprolific anemia with hematocrit around 30% -Normochromic, normocytic anemia, but sometimes hypochromic, microcytic -Bone marrow does not show erythroid hyperplastia or reticulocytosis -Normal or increased iron stores in bone marrow |
|
|
Describe the lab results for patients with malignancies. |
-In patients with malignancies, immature red and white cells can be seen -Abnormal red cell morphology -Low serum iron -Total iron binding capacity is decreased -Transferrin levels are decreased |
|
|
Describe treatment options for patients with anemia of inflammation. |
-Treatment of the underlying cause -If severe - blood transfusion -Iron therapy is NOT effective bc the patient is not utilizing iron effectively -Another option: erythropoeitin injections |
|
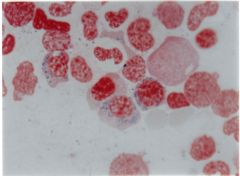
This type of anemia is associated with mitochondrial iron loading in the marrow erythroid precursors (ringed sideroblasts) and ineffective erythropoeisis.
The body has adequate iron, but it is unable to incorporate it into hemoglobin. Iron enters the developing red cell, but then accumulates in the mitochondria of the normoblast.
-There is a decrease in the enzyme delta ALA synthetase. |
Sideroblastic anemia |
|
|
What are some causes of sideroblastic anemia? |
-Congenital defects -Acquired defects -Malignant marrow disorders -Drugs -Toxins (alcohol or chronic lead poisoning) |
|
|
How is sideroblastic anemia diagnosed? |
-Variable red cell indices with microcytic hypochromic red cell picture -Increased serum iron and ferritin -Characteristic ringed sideroblast in bone marrow when stained for iron |
|
|
Describe the lab findings of sideroblastic anemia. |
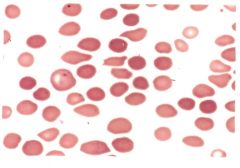
-Ringed sideroblast seen in bone marrow with a Prussian Blue stain -Increased erythtopoietic activity in bone marrow -Bone marrow will appear hypercellular, but the number of circulating reticulocytes is not elevated -Mature red cells are hypochromic with normocytic and microcytic cells (dimorphic population) -Target cells, basophilic stippling, dimorphic red cells are seen -10-40% of the nucleated red cells in the bone marrow are ringed sideroblasts -Leukocytes and platelets are usually normal -Iron and serum ferritin levels are increased |
|
|
This is a hereditary disorder of metabolism that produces inappropriately increased GI absorption of iron. It causes a progressive iron overload and excessive accumulation of iron in various organs. |
Hereditary hemochromatosis |
|
|
Describe the lab findings of hemochromatosis |
-Serum iron and total iron binding are increased -Ferritin levels are increased
|
|
|
Describe the treatment options for hemochromatosis. |
Therapeutic phlebotomy or iron chelation therapy |
|
|
Objectives of Hematoglobinpathies Powerpoint: |
-Describe hemoglobin defects and the effects of hemoglobinpathies that have a normal red cell function -Correlate lab data to the disease state -Describe lab findings in thalassemias |
|
|
What are the two most common hemoglobinpathies? |
Sickle cell anemia/trait and thalassemiaW |
|
|
What do most hemoglobinpathies arise from? |
Single amino acid substitutions |
|
|
What are the two ways in which these diseases can be inherited and how do they present in the child? |
-Autosomal dominant (will produce hemolytic disease in the heterozygous state) -Autosomal resessive (needs to be in the homozygous state to produce disease) |
|
|
What is the distinction between disease state and trait condition in hemoglobinopathies? |
Homozygous occurence of the gene (s/s) or possession of a heterozygous dominant gene that produces a hemolytic condition = DISEASE
Heterozygous and normally asymptomatic state (S/s) = TRAIT
In sickle cell anemia, the trait must be inherited from both parents in order to have the disease |
|
|
What are the percentages for normal adult hemoglobin? (A, A1, A2, F) |
Hb A - 95-98% Hb A1 - 3-6% Hb A2 - 2-3% Hb F - <1% |
|
|
What is sickle cell disease characterized by? |
-Production of Hb S -Anemia -Acute and chronic tissue damage secondary to the blockage of blood flow due to abnormally shaped cells |
|
|
What are common variants of sickle cell disease? |
-Hemoglobin SC disease -Beta-ThalassemiaT |
|
|
True or False: Every state in the US screens the blood of newborns for sickle cell disease |
True |
|
|
This is the cause of sickling on red blood cells. |
-When Hb S is deoxygenated, it becomes polymerized and produces sickling |
|
|
What is sickling promoted by? |
-Extremely reduced oxygen conditions (low oxygen tension) -Increased acidity in the blood (low pH) -Inreased 2,3-DPG -High cellular concentrations of hemoglobins -Loss of cell water -Hemoglobin C |
|
|
True or False: When sickled cells receive oxygen, they return to the normal shape |
True |
|
|
Repeated cycles of sickling and unsickling leads to permanent damage of the red cell. What are some side effects of this? |
Hemolysis, anemia, necrosis of body tissues due to lack of oxygen |
|
|
Describe the laboratory results for sickle cell anemia. |
-Hgb decreased -Hct decreased -Red cell count decreased -Persistent increase in wbc -Red cell morphology includes anisocytosis, poikilocytosis and hypochromia -Target cells, microcytes, polyhromatophilia, basophilic stippling -Sickle cells during crisis -Reticulocytes may be increased; increased MCV -Increased unconjugated bilirubin -Increased methamalbumin -Haptoglobin decreased -Increased LDH, AST -Increased urine urobilinogen |
|
|
List other diagnostic tests to determine if a patient has sickle cell anemia. |
-Sickle cell screening tests -Hemoglobin electrophoreisis -Alkali denaturation -Acid elution tests |
|
|
Describe the results of the hemoglobin electrophoresis test for a sickle cell disease patient. |
-Hemoglobin Electrophoresis: *80-95% Hgb S *0-20% Hgb F *Normal small amt of Hgb A2 |
|
|
How can newborns be screened for sickle cell disease? |
-Specimen obtained by heel stick -Cord blood -Electrophoresis at alkaline pH using cellulose acetate -Confirmation by electrophoresis at acid pH on citrate agar |
|
|
This type of sickle cell screening is used only on adults and children, not newborns. |
Metabisulfite sickle cell screening |
|
|
Describe methods to manage sickle cell disease. |
-Early detection and treatment of infections -Oral folate supplementation -Regular vaccines and check-ups -Treatment of pain crisis using: *Hydration *Analgesics *Oxygenation *Transfusion therapy *Hydroxyurea (stimulates Hb F production) |
|
|
This is a disorder in which the patient inherits a sickle gene from one parent and a gene for this disorder from the other parent. It results in severe hemolytic anemia. |
Sickle-Beta Thalassemia |
|
|
Describe blood smear results for Sickle-Beta Thalassemia. |
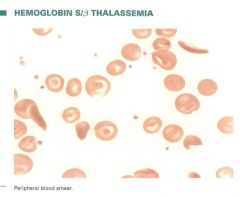
-Hypochromic, microcytic red cells with polychromatophilia -Target cells, stippling - rarely sickle cells -60-90% is Hb S -Splenectomy may be beneficial |
|
|
In this disease, patients only have HbS and HbC. Hgb A is absent, Hgb F is normal or increased. Mild anemia occurs in only 10% of patients and sickle cells are rarely seen. 50% of the cells are target cells and the spleen remains functional. Mild to moderate hemolysis. Target cells and plump sickle forms are seen on smears. |
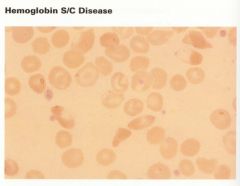
Sickle C disease |
|
|
This condition occurs when the patient inherits one HbS gene and one normal gene. With this condition, they have immunity to P. falciparum malaria. 35% of the cells contain HgbS. |
Sickle cell trait |
|
|
Thalassemia symptoms are caused by an abnormality in: |
The rate of synthesis of the globin chains, characterized by the absence or decrease in the synthesis of one of the two constituent globin subunits of the normal hemoglobin |
|
|
In this type of thalassemia, there is decreased synthesis of a certain type of alpha globulin. This results in the accelerated red cell destruction because of the formation of insoluble Hb H (excess beta chains) in red cells. |
Alpha thalassemia |
|
|
True or False: Beta thalassemia is more severe than alpha thalassemia. |
True; there is decreased beta globulin synthesis and alpha chains will precipitate, causing more problems with the beta chains |
|
|
Patents with Beta Thalassemia produce lots of this type of hemoglobin |
Hgb F |
|
|
Describe the laboratory findings for Beta Thalassemia. |
-Decreased hemoglobin -Decreased hematocrit -Decreased red cell count
Smears will show: -Anisocytosis -Poikilocytosis -Hypochromia -Target cells -Polychromatophilia -Nucleated red cells
-Microcytic -Hypochromic -MCV - Reduced -MCH - reduced -MCHC - reduced
-RDW increased due to anisocytosis -Reticulocytes are increased -Bilirubin and iron are increased -Ferritin is increased -Hemoglobin electrophoresis shows increased Hgb F and decreased Hgb A |
|
|
How can Beta Thalassemia be treated? |
-Regular blood transfusions, which may cause iron accumulation problems -Chelation therapy |
|
|
In this situation, only one abnormal gene is passed to the offspring. Patients can produce an adequate amount of alpha chains for hemoglobin synthesis. |
Silent carrier state of alpha thalassemia |
|
|
What are conditions for alpha thalassemia trait? |
-Two missing genes -Imbalance in production, Beta chains accumulate |
|
|
What occurs when a patient has Hemoglobin H disease? |
-Three gene deletions are present -Hb H is formed (4 beta chains) -Hemoglobin is nstable - precipitates in the red cells -Decreased cell life span due to membrane damage -Chronic, moderately severe anemia -Reticulocytes are increased, indices decreased -Hgb electrophoresis shows HbH, small amount of Hb Bart's with no HbA |
|
|
What are the symptoms of hydrops fetalis? |
-Four gene deletions -Total lack of alpha chains -Incompatible with life -Only Hgb produced is Hgb Barts (4 y chains) |
|
|
Describe the situation in the body when a patient is infected with Hemoglobin C disease. |
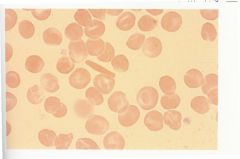
-Hb C differs from Hb A in one amino acid -When Hb C is deoxygenated, it tends to form intracellular crystals -Red cells are normochromic and normocytic -More than 50% of cells are target cells -Mild, chronic hemolytic anemia with splenomegaly |
|
|
What is the formula for calculating MCV? What is the normal value? What will cells look like if the value is normal, high, or low? |
MCV Formula: Hct(%) * 10 / RBC Count = fL
Normal Range: 80-96 fL Normal value: Normocytic Low value: Microcytic High value: Macrocytic
|
|
|
What is the formula for calculating MCH? What is the normal value? What will cells look like if the value is normal, high, or low? |
MCH Formula: Hgb (g/dl) * 10/ RBC Count = pg
Normal range: 27-32 pg Normal value: Normocytic Low value: Microcytic High value: Macrocytic |
|
|
What is the formula for calculating MCHC? What is the normal value? What will cells look like if the value is normal, high, or low? |
MCHC Formula: Hgb(g/dl) * 100 / Hct(%) = %
Normal Range: 32-36% Normal value: Normochromic Low value: Hypochromic High value: Hyperchromic |
|
|
What is the general rule for counting blood cells? |
Count cells touching the top and left of the square to prevent counting the same cells twice. |

