![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
70 Cards in this Set
- Front
- Back
|
Metabolism:
|
- the totality of an organism's chemical reactions.
- an emergent property of life that arises from interactions between molecules within the orderly environment of the cell. - as a whole, manages the material and energy resources of the cell. |
|
|
Metabolic Pathway:
|
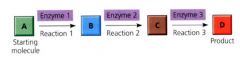
begins with a specific molecule, which is then altered in a series of defined steps, resulting in a certain product.
|
|
|
Catabolic Pathways:
|
pathways that release energy by breaking down complex molecules to simpler compounds
aka: breakdown pathways ex: cellular respiration in which glucose and other organic fuels are broken down in the presence of oxygen to carbon dioxide and water. Energy that was stored in the organic molecules become available to do work of the cell. |
|
|
anabolic pathways:
|
consume energy to build complicated molecules from simpler ones
aka: biosynthetic pathways ex: the synthesis of a protein from amino acids. |
|
|
Bioenergetics:
|
the study of how energy flows thorugh living organisms.
|
|
|
Energy:
|
- the capacity to cause change (do work)
- the ability to rearrange a collection of matter |
|
|
Kinetic energy:
|
- energy associated with the relative motion of objects
- moving objects can perform work by imparting motion to other matter. |
|
|
Heat/ Thermal energy:
|
kinetic energy associated with the random movement of atoms/ molecules.
|
|
|
potential energy:
|
energy that matter possesses because of its location or structure.
|
|
|
Chemical energy:
|
refers to the potential energy available for release in a chemical reaction.
|
|
|
Organism are ________ tranformers
|
energy
|
|
|
Transformations between potential and kinetic energy
|

|
|
|
Thermodynamics:
|
the study of the energy transformations that occur in a collection of matter
|
|
|
isolated system:
|
is unable to exchange either energy or matter within its surroundings.
|
|
|
open system:
|
energy and matter can be transferred between the system and its surroundings.
|
|
|
First law of Thermodynamics:
|
the energy of the universe is constant therefore energy can be transferred and transformed, but it cannot be created or destroyed.
aka: the principle of conservation of energy. |
|
|
Entropy:
|
- a measure of disorder, or randomness.
- the more randomly arranged a collection of matter is, the greater its entropy. |
|
|
Second Law of Thermodynamics:
|
every energy transfer or transformation increases the entropy of the universe.
for a process to occur spontaneuously, it must increase the entropy of the universe. |
|
|
The two laws of thermodynamics:
|

|
|
|
formula for Gibbs free energy of a system:
|
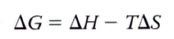
Delta H = the change in the system's enthalpy (in biological systems, equivalent to total energy)
Delta S = the change in the system's entropy T = the absolute temperature in Kelvin (K) units (K = C +273.15) |
|
|
Another formula (way) to think of Gibbs free energy:
|
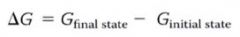
thus... delta G can be (-) only when the process involves a loss of free energy during the change from internal state to final state.
|
|
|
what is another term that describes maximum stability?
|
equilibrium
|
|
|
Think of free energy as a measure of a system's instability.. what is its tendency?
|
to change to a more stable state.
|
|
|
is -Delta G spontaneous or not spontaneous?
|
spontaneous
|
|
|
The relationship of free energy to stability, work capacity, and spontaneous change.
|
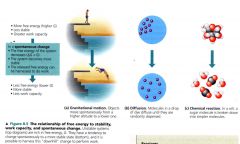
|
|
|
Exergonic:
|
energy outward
|
|
|
Endergonic:
|
energy inward
|
|
|
Exergonic Reaction:
|
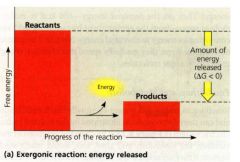
- proceeds with a net release of free energy.
- delta G is (-) because the chemical mixture loses free energy - magnitude of delta G = the max. amount of work the reaction can perform. |
|
|
Is cellular respiration an exergonic or endergonic reaction?
|
exergonic reaction
|
|
|
Endergonic Reaction:
|
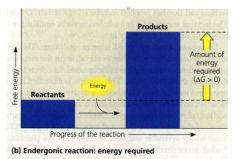
- one that absorbs free energy from its surroundings.
- b/c this kind of reaction essentially stores free energy in molecules delta G is (+) - magnitude of delta G = the quantity of energy required to drive the reaction. |
|
|
Is photosynthesis an exergonic or endergonic reaction?
|
endergonic reaction.
|
|
|
Equilibrium and work in isolated and open systems
|
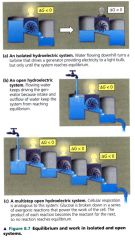
|
|
|
Energy coupling:
|
the use of an exergonic process to drive an endergonic one.
|
|
|
ATP
|
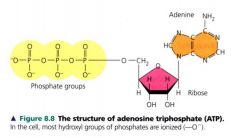
Adenosine triphosphate
- contains the sugar ribose w/ the nigrogenous base adenine & a chain of 3 phosphate groups bonded to it. - one of the nuceloside triphospahtes used to make RNA |
|
|
The hydrolysis of ATP:
|
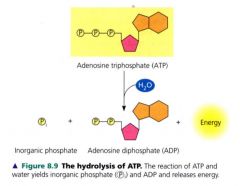
The bonds between the phosphate groups of ATP can be broken by hydrolysis. The terminal phosphate bone is broken, a molecule of inorganic phosphate leave the ATP, which becomes adenosine diphosphate (ADP).
the reaction is exergonic and releases 7.3kcal of energy per mole of ATP hydrolyzed. |
|
|
What are the 3 main kinds of work a cell does?
|
- chemical work
- transport work - mechanical work |
|
|
Phosphorylated:
|
the recipient of the phosphate group from the hydrolysis of ATP.
|
|
|
what is the key to coupling exergonic and endergonic reactions?
|
the formation of the phosphorylated intermediate, which is more reactive (less stable) than the original unphosphorylated molecule.
|
|
|
How ATP drives chemical work: Energy coupling using ATP hydrolysis
|

|
|
|
How ATP drives transport and mechanical work:
|
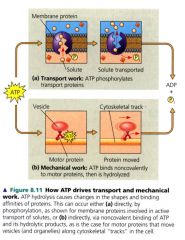
|
|
|
The ATP cycle:
|
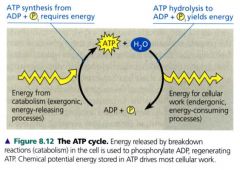
- ATP is a renewable resource that be be regenerated by the addition of phosphate to ADP.
- Catobolic (exergonic) pathways, especially cellular respiration, provide the energy for the endergonic process of making ATP. - the chemical potential energy temporarily stored in ATP drives most cellular work. |
|
|
Phosphorylation:
|
the addition of phosphate groups
|
|
|
Example of an enzyme-catalyzed reaction: hydrolysis of sucrose by sucrase
|
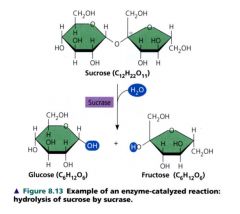
|
|
|
Enzyme:
|
A macromolecule that acts as a catalyst
|
|
|
Catalyst:
|
a chemical agent that speeds up a reaction without being consumed by the reaction.
|
|
|
Activation Energy:
|
aka: free energy of activation
- the initial investment of energy for starting a reaction - the energy required to contort the reactant molecules so the bonds can break. - the amount of energy need to push the reactants over an energy barrier (hill) so that the "downhill" part of the reaction can begin. |
|
|
Energy Profile of an exergonic reaction:
|
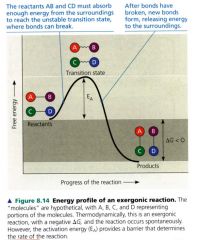
|
|
|
How does the absorption of thermal energy increase the speed of the reactant molecule?
|
the molecules collide more often and more forcefully making the bonds more likely to break.
|
|
|
how does an enzyme catalyze a reaction?
|
by lowering the activation energy barrier enabling the reactant molecules to absorb enough energy to reach the transition state even at moderate temperatures.
|
|
|
how do enzymes determine which chemical process will be going on in the cell at any particular time?
|
b/c they are very specific for the reactions they catalyze.
|
|
|
The effect of an enzyme on activation energy
|
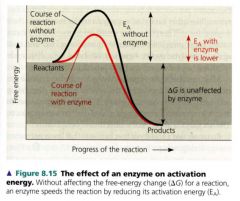
|
|
|
Enzyme's substrate:
|
the reactant an enzyme acts on.
|
|
|
most enzyme's names end in -___?
|
-ase
|
|
|
what accounts for molecular recognition in enzymes?
|
recall: enzymes are proteins and proteins are macromolecules w/ unique 3D configurations.
the specificity of an enzyme results from its shape which is a consequence of its amino acid sequence. |
|
|
Active site:
|
restricted region of the enzyme molecule that binds to the substrate
- is typically a pocket or groove on the surface of the protein where catalysis occurs. |
|
|
The active site and catalytic cycle of an enzyme:
|
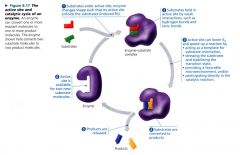
|
|
|
What is a saturated enzyme?
|
when the concentration of substrate is high enough that all enzyme molecules have their active sites engaged.
as soon as the product exits an active site, another substrate molecule enters. |
|
|
how do cells increase the rate of reaction?
|
by producing more enzyme molecules.
|
|
|
Environmental factors affecting enzyme activity
|
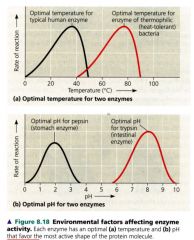
|
|
|
cofactor:
|
non protein helpers that enzymes require for catalytic activity.
|
|
|
coenzyme:
|
a cofactor that is an organic molecule.
|
|
|
Competitive Inhibitors:
|
- resemble the normal substrate molecule & compete for admission into the active site.
- reduce the productivity of enzymes by blocking substrates from entering active sites. |
|
|
noncompetitive inhibitors:
|
- do not directly compete w/ the substrate to bind to the enzyme at the active site.
- they impede enzymatic reactions by binding to another part of the enzyme. - interaction causes enzyme molecules to change its shape in such a way that the active site becomes less effective at catalyzing the conversion of substrate to product. |
|
|
Inhibition of enzyme activity
|
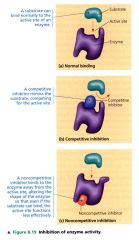
|
|
|
Allosteric Regulation:
|
any case in which a protein's function at one site is affected by the binding of a regulatory molecule to a separate site.
- result in either inhibition or stimulation of an enzyme's activity. |
|
|
an activating or inhibiting regulatory molecule binds to a regulatory site sometimes also called what?
|
an allosteric site.
|
|
|
Cooperativity:
|
this mechanism amplifies the response of enzymes to substrates
- one substrate molecule primes an enzyme to accept additional substrate molecules more readily. |
|
|
Allosteric activators and inhibitors
|
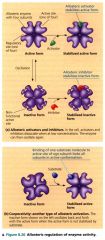
|
|
|
INQUIRY: Are there allosteric inhibitors of caspase enzymes?
|

|
|
|
Feedback Inhibition:
|
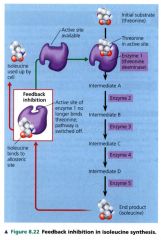
a metabolic pathway is switched off by the inhibitory binding of its end product to an enzyme that acts early in the pathway.
- prevents the cell from wasting chemical resources by making more than is necessary. |

