![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
|
What is a disaccharide? |
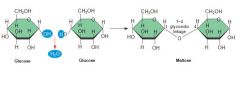
A sugar molecule consisting of TWO monosaccharides linked by a dehydration reaction |
|
|
What is meant by saying that something is hydrophilic or hydrophobic? |
Hydrophilic - "water loving"; pertaining to polar or charged molecules (or parts of molecules) that are soluble in water
Hydrophobic = "water-fearing" pertaining to nonpolar molecules (or parts of molecules) that do not dissolve in water). |
|
|
What is a nucleic acid? What are the two types of nucleic acids? |
A polymer consisting of many nucleotide monomers; serves as a blueprint for proteins and through actions of proteins, for all cellular structures and activities. The two types of nucleic acids are DNA and RNA. |
|
|
What is an unsaturated fatty acid |
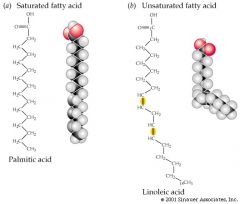
A fatty acid that has one or more double bonds between carbons (causing KINKS) in the hydrocarbon tail and thus lacks the maximum number of hydrogen atoms.
Unsaturated fats and fatty acids do not solidify at room temperature |
|
|
What is a carbohydrate? |
Member of the class of biological molecules consisting of single monomer sugars (i.e. monosaccharides), two-monomer sugars (I.e. disaccharides), and polymers (polysaccharides) |
|
|
What is an organic compound? And provide one example |
An organic compound is a chemical compound containing the element carbon and usually the element hydrogen
An example is a hydrocarbon, which is an organic compound composed of only the elements carbon and hydrogen
|
|
|
Define a fat, and what its function is |
A fat is a lipid composed of three fatty acids linked to one glycerol molecule; a triglyceride. Most fats function as energy-storage molecules. |
|
|
What is a polymer? |
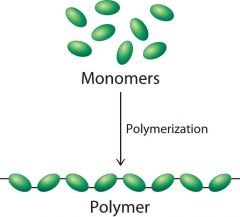
A large molecule consisting of many identical or similar monomers linked together by covalent bonds. |
|
|
What is cellulose and what types of bonds are they linked by? |
A structural polysaccharide of plant cell walls composed of glucose monomers.
Cellulose molecules are linked by hydrogen bonds into cable-like fibrils. |
|
|
What is a polysaccharide? |
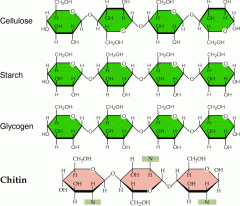
A carbohydrate polymer of many monosaccharides (sugars) linked by dehydration reactions. |
|
|
What is a monomer ? |
The subunit that serves as a building block of a polymer. |
|
|
What is an enzyme and what is its function? |
An enzyme is a macromolecule, usually a protein, that serves as a biological catalyst, changing the rate of a chemical reaction without being consumed by the reaction. |
|
|
What is a carboxyl group |
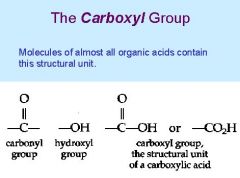
A chemical group consisting of a carbon atom double-bonded to an oxygen atom and also bonded to a hydroxyl group |
|
|
What is a starch? |
A storage polysaccharide in plants; a polymer of glucose. |
|
|
What is a carbonyl group? |
A chemical group consisting of a carbon atom linked by a double bond to an oxygen atom |
|
|
What is an amino acid |
An organic molecule containing a carboxyl group and an amino group; serves as the monomer of proteins |
|
|
What is a functional group? |
A specific configuration of atoms commonly attached to the carbon skeletons of organic molecules and involved in chemical reactions. |
|
|
What are the three levels of a protein? |
1. primary structure - 1st level, specific sequence of amino acids making up a polypeptide chain 2. secondary structure - the regular local patterns of coils or folds of a polypeptide chain 3. tertiary structure - 3rd level - the overal 3-d shape of a polypeptide due to interactions of R groups of the amino acids making up the chain. 4. quarternary structure - the 4th level of a protein, the shape resulting from the association of two or more polypeptide subunits. |
|
|
What are isomers? |
Organic compounds with the same molecular formula but different structures and therefore, different properties. |
|
|
What is a monosaccharide? |
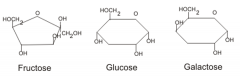
The simplest carbohydrate; a simple sugar with a molecular formula that is generally some multiple of CH2O. Monosaccharaides are the monomers of disaccharides and polysaccharides. |
|
|
What is a nucleotide? |
A building block of nucleic acids, consisting of a five-carbon sugar covalently bonded to a nitrogenous base and one more more phosphate groups. |
|
|
What is the methyl group? |
A chemical group consisting of a carbon atom bonded to 3 hydrogen atoms. |
|
|
What are phospholipids? |
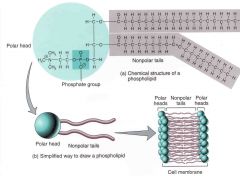
A lipid made up of glycerol joined by two fatty acids and a phosphate group, giving the molecule two nonpolar hydrophobic tails and a polar hydrophilic head.
Phospholipids form bilayers that function as biological membranes. |
|
|
What is the phosphate group? |
A chemical group consisting of a phosphorous atom bonded to four oxygen atoms. |
|
|
What is a macromolecule and what are the three formed by dehydration reactions? |
A giant molecule formed by the joining of smaller molecules, usually by dehydration: 1. a protein, 2. a carbohydrate, 3. a nucleic acid |
|
|
What is glycogen? |
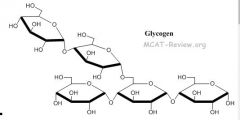
An extensively branched glucose storage polysaccharide found in liver and muscle cells; the animal equivalent of starch. |
|
|
What is a trans fat? |
An unsaturated fat linked to health risks that is formed artificially during the hydrogenation of vegetable oils. |
|
|
What is RNA (what does it stand for) and what are the four nitrogenous bases? |
RNA stands for ribonucleic acid - it is a type of nucleic acid consisting of nucleotide monomers with a ribose sugar and the nitrogenous bases A (adenine), C (cytosine), G (guanine) and U (uracil); usually single-stranded; functions in protein synthesis, gene regulation, and as the genome of some viruses |
|
|
What is a steroid and name three examples |
A type of lipid whose carbon skeleton is in the form of four fused rings with various chemical groups attached. Examples include: cholesterol, testosterone, and estrogen |
|
|
What is cholesterol? |
A steroid that is an important component of animal cell membranes and that acts as a precursor molecule for the synthesis of other steroids, such as hormones. |
|
|
What is a lipid? Is it hydrophobic of hydrophilic?
List three examples. |
An organic compound consisting of mainly carbon and hydrogen atoms linked by nonpolar covalent bonds, making the compound mostly hydrophobic. Lipids include fats, phospholipds, and steroids and are insoluble in water |
|
|
What is a double helix? |
The form of native DNA, referring to its two adjacent polynucleotide strands interwound into a spiral shape. |
|
|
Describe denaturation and some causes of it |
A process in which a protein unravels, losing its specific structure and hence function; can be caused by changes in pH or salt concentration or by high temperature;
Denaturation also refers to the separation of the two strands of the DNA double helix; caused by similar factors |
|
|
What is the amino group? |
A chemical group consisting of a nitrogen atom bonded to two hydrogen atoms. |
|
|
What is a protein? |
A functional biological molecule consisting of one or more polypeptides folded into a specific 3-D structure |
|
|
What is a peptide bond? |
The covalent bond between two amino acid units in a polypeptide, formed by a dehydration reaction. |
|
|
What is a gene? |
The simplest unit of heredity.
Consisting of specific nucleotide sequence in DNA (or RNA, in some viruses). Most of the genes of a eukaryote are located in its chromosomal DNA; a few are carried by the DNA of mitochondria and chloroplasts. |
|
|
What is chitin? |
A structural polysaccharide found in many fungal cell walls and in the exoskeletons of arthropods. |
|
|
What is the hydroxyl group? |
A chemical group consisting of an oxygen atom bonded to a hydrogen atom |
|
|
What are the four levels of protein structure? (Describe images) |
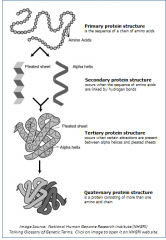
|
|
|
What is a saturated fatty acid? |
A fatty acid in which all carbons in the hydrocarbon tail are connected by single bonds and the maximum number of hydrogen atoms are attached to the carbon skeleton. Saturated fats and fatty acids solidify at room temperature. |
|
|
What is glucose? |
A six-carbon monosaccharide that serves as a building block for many polysaccharides and whose oxidation in cellular respiration is a major source of ATP for cells. |
|
|
What is a polypeptide? |
A polymer (chain) of amino acids linked by peptide bonds. |
|
|
What is an anabolic steroid? |
A synthetic variant of the male hormone testosterone that mimics some of its effects |
|
|
Describe a dehydration reaction vs hydrolysis |
dehydration reaction A chemical reaction in which two molecules become covalently bonded to each other with the removal of a water molecule
hydrolysis - a chemical reaction that breaks bonds between two molecules by the addition of water; process by which polymers are broken down and an essential part of digestion. |
|
|
What are the six important chemical groups, which 5 are functional, which one is |
1. hydroxyl group (-OH) consists of a hydrogen atom bonded to an oxygen atom, which in turn is bonded to the carbon skeleton. Organic compounds containing hydroxyl groups are called alcohols (e.g. ethanol) 2. Carbonyl group (>C=O) a carbon atom is linked by a double bond to an oxygen atom. If the carbonyl group is at the end of a carbon skeleton it is referred to as an aldehyde; if it is within the chain, the compound is called a ketone 3. Carboxyl group (-COOH) consists of a carbon double bonded to an oxyen atom and also bonded to a hydroxyl group. Acts as an acid by contributing an H+ to a solution 4. Amino group (-NH2) has a nitrogren bonded to two hydrogens and the carbon skeleton. It acts as a base by picking up an H+ from a solution. organic compounds with an amino group are called amines. The building blocks of proteins - amino acids - contain an amino and a carboxyl group 5. Phosphate group (-OPO3^2-) consists of a phosphorous atom bonded to four oxygen atoms. It is usually ionized and attached to the carbon skeleton by one of its oxygen atoms. Compounds with phosphate groups are called organic phosphates and are often involved in the transfer of energy 6. The sixth group, a methyl group (-CH3) is nonpolar and not reactive but it affects molecular shape and thus function. It consists of a carbon bonded to three hydrogen atoms. |
|
|
The reaction that joins two monomers to form a polymer is known as a __________reaction. The molecule of water formed is due to the interaction between ___________ and a hydrogen ion |
dehydration.... a hydroxyl group |
|
|
Double bonds are present in the majority of the fats of which of the following foods?
margarine, peanuts, red steak |
Peanuts. Remember unsaturated fatty acids have double bonds. (b/c they have kinks so can be fully saturdated). |

