![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
First Line of defense against pH changes
|
Chemical Buffer System:
Bicarbonate Phosphate Protein |
|
|
Second Line of Defense against pH changes
|
Physiological Mechanisms:
Respiratory System (regulates PCO2) Renal System (regulates HCO3) |
|
|
Renal response to Acidosis
|
When pH falls, there is increased proton secretion in urine and urine becomes acidic and blood basic. Phosphate and ammonia buffers are used and excreted
|
|
|
Renal Response to Alkalosis
|
When pH decreases, there is increased HCO3- excretion, and urine becomes alkaline (basic) while blood becomes acidic.
|
|
|
Normal Levels by acid base analyzers
|
pH ; 7.36 -7.44
PCO2 : 38 - 42 mmHG HCO3-: 22 - 25 mmol/L |
|
|
Respiratory Acid Base Disorders
|
Due to dysfunction of the respiratory system and are result of changes in PCO2
|
|
|
Metabolic Acid Base Disorders
|
Due to metabolic or renal disorders and caused by changes in HCO3-
|
|
|
Respiratory Acidosis
|
Decreased rate of respiration/lung function or obstruction (hypoventilation)
-CO2 not wash out, increased in PCO2 Will cause a decrease in pH, elevated PCO2, with normal HCO3_ |
|
|
Compensation for respiratory acidosis
|
Compensation will by the renal system until primary disturbance is treated. pH will slightly increase to try to return to normal, PCO2 will stay elevated. HCO3 will increase by the renal system as the kidney tries excrete H+ ions to increase pH. Phosphate and ammonia will acidify the urine.
|
|
|
Henderson-Hasselbalch Respiratory Acidosis
|
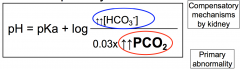
Increase in PCO2 results in decrease in pH
Compensation by renal system, increases HCO3- and ratio of acid to base return almost to normal |
|
|
Cause of respiratory acidosis
|
-Airway obstruction
-Opiod drugs - anesthetics -Muscle diseases in chest wall -Disease/injury of phrenic nerve (Gullian Barre syndrome) -Lung disease, COP@, RDS, fibrosis of lung |
|
|
Respiratory Alkalosis
|
Increase rate of respiration --> increase washout of CO2, resulting in low PCO2 ---> increasing pH
-pH is increased -PCO2 is low -HCO3 is normal |
|
|
Compensation of respiratory Alkalosis
|
Compensation will be by the renal system as it tries to bring pH back to normal by not excreting H+, resulting in increased excretion of HCO3- in the urine (causing urine) to become alkaline.
pH increases closer to normal PCO2 - remains low HCO3 - decreases due to renal compensation |
|
|
Henderson-Hasselbalch Respiratory Alkalosis
|
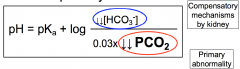
Decrease in PCO2 (due to hyperventilation) results in increased in pH
HCO3- decreases due to renal compensation and ratio of base/acid returns toward normal |
|
|
Causes of respiratory alkalosis
|
Causes of hyperventilation:
-Anxiety, fever -Hypoxia - high altitude stimulates increase rate of respiration |
|
|
Metabolic Acidosis
|
Low HCO3- (base) results in low pH
HCO3- is low either due to increased nonvolatile acids (lost by buffering) or increases losses -pH is decreased -PCO2 is normal -HCO3 is decreased |
|
|
Compensation of Metabolic Acidosis
|
When plasma pH falls, stimulates respiration to washout CO2 and decreased PCO2-
If renal system is functioning it can compensate to increase H+ excretion -pH is closer to normal -PCO2 decreased due to compensatory hyperventilation -HCO3- decreased |
|
|
Causes of Metabolic Acidosis
|
Increased production of non-volatide acids
Increased loss of HCO3- (base) |
|
|
Diabetic Ketoacidosis
|
Results in increased production of non-volatile ketone bodies
|
|
|
Lactic Acidosis
|
Results in increased production of non-volatile lactate
|
|
|
Chronic Renal Failure
|
Results in metabolic acidosis due to decreased excretion of sulfate and phosphate
|
|
|
Renal tubular acidosis
|
-Causes metabolic acidosis due to failure to secret h+ and reabsorb HCO3-
|
|
|
Metabolic Alkalosis
|
Results from increased HCO3- causing increase in pH.
-pH is increased -HCO3 is increased -PCO2 is normal |
|
|
Compensated metabolic alkalosis
|
Increased pH inhibits respiratory center resulting in hypoventilation --> increases PCO2
-If renal system is function, can increase excretion of HCO3- -pH comes closer to normal -HCO3- is increased (primary abnormality) -PCO2 is increased due to respiratory compensation |
|
|
Causes of Metabolic alkalosis
|
Vomiting, pyloris stenosis
-results in loss of acidic contents of stomach Nasogastric suction - draining stomach Excess consumption of antacids |
|
|
Summary
|
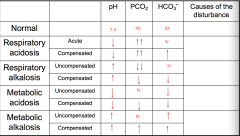
|

