![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
39 Cards in this Set
- Front
- Back
|
What are the enzymes and coenzymes required for PDH Complex?
|
3 enzymes:
1) Pyruvate Dehydrogenase 2) Dihydrolipoyl Transacetylase 3) Dihydrolipoyl Dehydrogenase 5 co-enzymes 1) Co-ASH 2) NAD+ 3) FAD+ 4) Lipoic Acid 5) TPP (Thymine Pyrophosphate) |
|
|
What are the end products of PDH complex and where does it occur?
|
Pyruvate ---> Acetyl-CoA + NADH + H+
Occurs in the matrix of the mitochondria. |
|
|
What is responsible for activation PDH and what signals cause activation?
|
Activated when dephosphorylated by endogenous phosphatase.
Signals - promote dephosphorylation - insulin in adipocytes - catecholamine in cardiac muscle - calcium in skeletal muscle |
|
|
What is responsible for the inhibition of PDH?
|
Enzyme PDH is inhibited when it is phosphorylated.
Molecules that promote this through protein kinase - Acetyl CoA - ATP - NADH These are products and can inhibit. |
|
|
What are other sources of Acetyl-CoA?
|
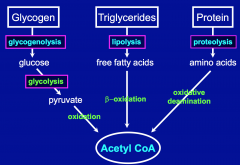
B-oxidation of FFA (produced from lipolysis of triglycerides)
Oxidative Deamination of AA (produced from proteolysis of proteins) |
|
|
What are the 4 enzymes in TCA cycle that are regulated?
|
Citrate synthase
Isocitrate dehydrogenase a-KG dehydrogenase Succinate dehydrogenase |
|
|
What activates and inhibits citrate synthase?
|
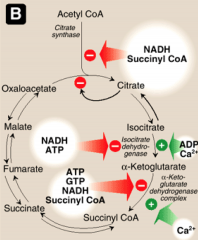
Activated by substrate availability (acetyl-CoA and oxaloacetate), calcium and ADP.
Inhibited by high ATP, NADH, succincyl CoA and Fatty Acyl coA (high energy molecules) General mechanism: Low energy - activated High energy - inactivated |
|
|
What activates and inhibits isocitrate DH?
|
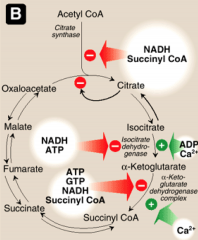
Activated by ADP and calcium.
Inhibited by NADH and ATP. |
|
|
What activates and inhibits a-KG DH?
|
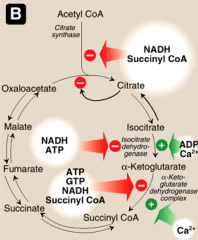
Activated by Calcium.
Inhibited by ATP, GTP, NADH and succinyl CoA. |
|
|
What activates and inhibits succinate DH?
|
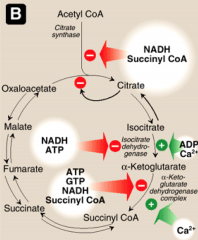
Activated by ADP, Pi, and succinate (substrate)
Inhibited by OAA (oxaloacetate) |
|
|
What is PDH deficiency and what are the affects?
|
Will results in high pyruvate resulting in high lactic acid and alanine. Result in low production of acetyl CoA and severe reduction in ATP production.
Causes lactic acidosis, neurologic defects, myopathy and is usually fatal. |
|
|
What is Werneke-Korsakoff Syndrome?
|
It thiamine deficiency which is characterized by ataxia, ophthalmolplagia, memory loss and cerebral hemorrhage.
Alcoholics and malnourished individuals are at risk. Can result in heart failure, decrease ATP and increased cardiac a-KG DH and a-ketoacid DH require thymine. |
|
|
What affect does Arsenic poisoning have on PDH?
|
Arsenic binds to lipoc acod which is needed for dihydrolipoyl transacetylase in PDH complex. Results in lactic acidosis, neurological defects and can lead to death.
Affects other lipoic acid requiring enzymes like a-ketogluterate DH and a-ketoacid DH. |
|
|
What is the affect of Fluoracetate?
|
Inhibits aconitase - enzyme that is responsible for conversion of citrate to isocitrate. Inhibits entire TCA cycle.
|
|
|
What vitamin deficiencies can affect the TCA cycle?
|
Niacin (B3 - needed for NAD)
Riboflavin (B2 - needed for FAD) Thiamine (most important - needed for TPP) Pantothenate -(CoA - from vitamin B5) Cobalamin (B12) (has to to with production of succinyl-CoA) |
|
|
What is affect of Malonate?
|
Inhibits succinate dehydrogenase (enzymes responsible for conversion of succinate to fumarate) in TCA cycle.
|
|
|
What is Leber's Hereditary Optic Neuropathy?
|
It is a defect in NADH dehydrogenase (ETC). Progressive retinal degeneration. Defect in one of the mitochondrial enzymes.
|
|
|
What are ragged red fibers?
|
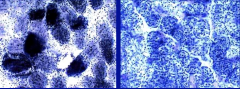
This is a succinic DH (SDH) stain that is used to diagnose mitochondrial disorder. Results in clumpiness that shows disorder for mitochondria.
|
|
|
What is the energy given from ATP, ADP and AMP?
|

|
|
|
What are the 4 protein complexes and what are the enzymes involved?
|
Complex I = NADH dehydrogenase
Complex II = Succinate dehydrogenase Complex III = Cytochrome reductase Complex IV = Cytochrome oxidase 2 Electron Carriers: Coenzyme Q: moves between 1-3, and 2-3 Cytochrome C: moves between 3-4 |
|
|
What are the prosthetic groups for the protein complexes in ETC?
|
Complexes I and II:
• FMN and FAD (riboflavin derivative) Complex III • heme group (Fe3+) Complex IV • Cu2+ and heme group (Fe3+) Also Iron-sulfur proteins, present in complexes I, II, and III. |
|
|
Where does NADH come from for complex 1?
|
NADH electrons come from from malate dehydrogenase (MDH), α-Ketoglutarate dehydrogenase (a-KGDH), isocitrate dehydrogenase (IDH), pyruvate dehydrogenase (PDH), as well as from fatty acid β- oxidation and from cytosolic sources such as glycolysis.
Most NADH will enter at Complex 1. |
|
|
Where does FADH come from for complex II?
|
FADH2 electrons from succinate dehydrogenase (SDH) or the associated CoQ gets them from glycerol
phosphate shuttle or from fatty acid β-oxidation. Most FADH2 will enter at Complex II. |
|
|
What is the first step of ETC?
|
1) NADH is oxidized by CoQ at complex 1
and/or FADH2 is oxidized by CoQ at complex 2 |
|
|
What happens to CoQH2?
|
It is oxidized by Cytochrome C reductase at Complex 1 and cytochrome C is oxidized by O2 at complex IV.
|
|
|
What is Amytal Rotenone and piericidin A?
|
Rotenone, piericidin A (bacterial antibiotic) and the barbiturate amytal inhibit NADH dehydrogenase in complex I
|
|
|
What is Antimycin A and how does it affect ETC?
|
Antimycin A (an antibiotic) inhibits cytochrome b of cytochrome reductase (complex III)
|
|
|
What is are inhibiters of cytochrome oxidase (complex IV)?
|
CO, azide, Hydrogen Sulphide (H2S) and cyanide (CN-) inhibit cytochrome oxidase (complex IV)
|
|
|
What is oligomycin?
|
Oligomycin (a streptomyces antibiotic) inhibits ATP synthase.
|
|
|
What is the function of Adenine nucleotide translocase?
|
Protein that has anti-port of ATP and ADP, to allow ATP to be transported back to the cytosol of the cell.
|
|
|
What is atractyloside?
|
Is a toxic glycoside from thistle plant Atractylis gummifera
- Binds the outward facing (inter- memb space) portion of the adenine nucleotide transporter |
|
|
What is Bongkrekic Acid?
|
Is a respiratory toxin produced in coconuts contaminated with Burkholderia gladioli
Binds the inward facing (matrix) portion of the adenine nucleotide transporter |
|
|
Thermogenin
|
Is an uncoupler of oxidative phosphorylation.
It causes dissipation of H+ gradient and generates heat. This is the function of brown adipose tissue. Brown -> abundance of cytochrome- containing mitochondria. |
|
|
What are some uncouplers of oxidative phosphorylation?
|
DNP (dinitriphenol)
ASA (asipirin) Thermogenin Inophores |
|
|
What is uncoupling of oxidative phosphorylation?
|
Involves uncoupling of ATP synthase which results in protein leak of H+ back into the matrix disrupting the gradient. Punch holes into the membrane.
Decrease ATP synthesis, increase ETC and oxygen consumption. |
|
|
What is the affect of Ionophores on oxidative phosphorylation?
|
They are compounds that make the inner membrane permeable to compounds that usually cannot cross. They will increase the proton permeability - resulting gin dissipation of proton gradient.
Can be channel formers (make a pore) or mobile carriers. |
|
|
What is gramicidin?
|
It is a Ionophore that forms channels. It is a mixture of antibiotic produced by Bacillus brevis.
Dissipate protein gradient. |
|
|
What is Valinomycin?
|
It is a Ionophore, that is mobile carrier and an antibiotic from strains from streptomyces strains. Typically associated with carrying K+.
Dissipate protein gradient. |
|
|
What is the affect of hypoxia?
|
Low O2 can slow down the rate ETC, because it is the final acceptor in the eTC. Can result in MI.
|

