![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
55 Cards in this Set
- Front
- Back
|
What is the hierarchy of atoms to organisms?
|
1. Atoms
2. Molecules 3. Macromolecules 4. Macromolecular Assemblies 5. [Organelles] 6. Cells 7. [Tissues] 8. Organisms |
|
|
What are the 2 major types of cells?
|
Prokaryotes & Eukaryotes
|
|
|
Eukaryotes: 4 characteristics & Example
|
1. No nucleus
2. No organelles 3. Single-Circular Chromosomes 4. Divide Quickly Ex.) Bacteria |
|
|
Prokaryotes: 4 characteristics & Example
|
1. Has Nucleus
2. Has Organelles 3. Multiple-Linear Chromosomes 4. Longer to Divide |
|
|
Organelles
|
- Discrete, membrane bound compartment within a cell with a discrete function
- Not all cells have organelles in equal amounts: the type and # reflect function |
|
|
Nucleus
|
DNA replication & transcription
|
|
|
Nucleolus
|
Not an organelle; makes ribosomes
|
|
|
Mitochondria
|
Citric acid cycle, beta oxidation, oxidative phosphorylation, generate ATP
|
|
|
Rough Endoplasmic Reticulum (RER)
|
Directs proteins (secreted or membrane) to location
|
|
|
Golgi Body
|
Carbohydrates are attached to proteins (only secreted/membrane)
Ex) Blood proteins - A carb, B carb, both, or none |
|
|
Smooth Endoplasmic Reticulum (SER)
|
Fat metabolism
|
|
|
Lysosome
|
Breaks down macromolecules
|
|
|
Macromolecular Assemblies
|
Made of macromolecules not held by a covalent bond
Ex) Protein & DNA |
|
|
What are the most common atoms in organisms?
|
C, H, O, N
|
|
|
Functional Groups: Carbon-Carbon
|
1. Alkane
2. Alkene (trans or cis) |
|
|
Functional Groups: Carbon-Oxygen
|
1. Alcohol
2. Ketone 3. Aldehyde 4. Carboxylic Acid 5. Ester 6. Ether |
|
|
Functional Groups: Carbon-Nitrogen
|
1. Amide
2. Amine |
|
|
Amino Acids
|
Macromolecule: protein
How Big: hundreds of a.a. Bond: peptide Function: enzymes, communication (ex. Insulin), transport (ex. Hemoglobin), structure (ex. Collagen), defense (ex. Antibody), other |
|
|
Draw Amino Acids & Protein
|
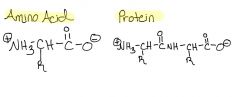
|
|
|
Sugars
|
3 common 6 carbon sugars: glucose, fructose, galactose
Macromolecule: glycogen, starch (animal); cellulose (plant) Bond: alpha 1-4 ether bond (glucose) |
|
|
Draw Glucose & Starch
|
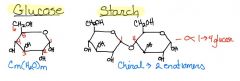
|
|
|
Nucleotide
|
- Nitrogenous base (A,G,C,T,U) + 5 carbon sugar (Ribose or Deoxyribose)
Macromolecule: DNA (Deoxyribose) & RNA (Ribose) DNA + Phosphate + A/C/T/G RNA + Phosphate + A/C/U/G - DNA Function: stores genetic info (3 nucleotides encode 1 a.a.) - RNA Function: intermediate in flow of genetic info |
|
|
Draw a Nucleotide & DNA
|
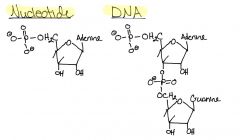
To form macromolecule, phosphate of incoming nucleotide attaches to 3' OH of sugar
|
|
|
Simple Fats
|
Fatty Acids: Saturated or Unsaturated (Double Bonded Carbons)
Bonds: Ester, phosphoester, amide, ether Macromolecules: 1.TAG (energy storage) 2. PL (membrane) 3. Sphingolipid (membrane) 4. Cholesterol (stiffens membrane, starting point for synthesis of steroid hormones) |
|
|
Draw Saturated & Unsaturated Fatty Acids
|

|
|
|
What is the problem with trans fat?
|
1. Can be catabolized
2. Tend to raise LDL (bad cholesterol) and lower HDL (good cholesterol) |
|
|
Difference b/w Cis & Trans
|
- All naturally occurring fats are cis
- Trans fats are made by hydrogenation of naturally occurring fats |
|
|
What are omega fats?
|
Omega number is number of carbons in 1st double bond from non-carboxylic acid end
|
|
|
Triacylglycerol (TAG)
|
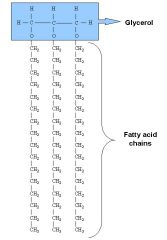
Glycerol + 3 fatty acids
[Ester Bond] |
|
|
Phospholipid (PL)
|
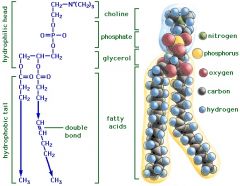
Glycerol + 2 fatty acids + phosphate + "x"
[Ester (fatty acids) & Phosphoester Bonds (phosphate)] |
|
|
Sphingolipid
|
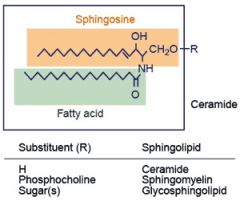
Sphingosine + fatty acid + sugar
[Amide (Fatty acid) & Ether (Sugar) Bonds] |
|
|
Cholesterol
|
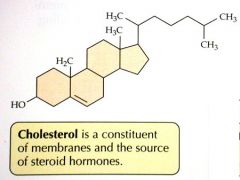
|
|
|
What is metabolomics & how is it done?
|
Separate & ID all molecules in any given cell
How? 1. High powered NMR 2. Gas Chromoatography-Mass Spectrometry (GC) |
|
|
Process of Metabolomics
|

|
|
|
Acid & Base: Ka
|
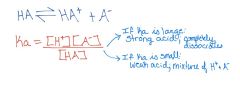
|
|
|
Acid & Base: pKa
|
- Log of Ka
- Meaning: pH at which 50% HA and 50% A- |
|
|
Henderson-Hasselbach
|
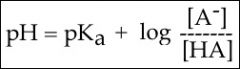
Calculates ratio of acid to base
Use to find % acid & % base to calculate overall charge |
|
|
How to calculate charge
|
1. Find % acid and % base
2. Draw acid form & base form to find individual charges (0, +1, -1) 3. Multiply charges by percentages & add Charge = %A(charge) + %HA(charge) |
|
|
% HA and % A- when pH is ______ than pKa. (3 less, 2 less, 1 less, equal, 1 more, 2 more, 3 more)
|
3 < : 99.9% HA, 0.1% A-
2 < : 99% HA, 1% A- 1 <: 90% HA, 10% A- equal: 50% HA, 50% A- 1 >: 10% HA, 90% A- 2 >: 1% HA, 99% A- 3 >: 0.1% HA, 99.9% A- |
|
|
Rule of thumb w/ pKa & pH
|
pH < pKa: acid
pH +1 or -1 from pKa: mixture pH > pKa: base |
|
|
Titration Curve
|
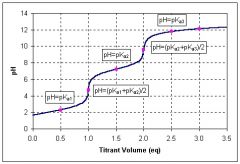
Flat = buffer regions
Steep = equivalence points Titrant: NaOH |
|
|
Draw Amino Acid Backbone at pH 2, 7, 11
|
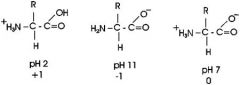
Amine group: pKa=9
Carboxylic Acid group: pKa=2 R group: variable |
|
|
Are amino acids good physiological buffers?
|
Physiological pH is 7.
Unless R group has a pKa around 7, a.a. are not good buffers. |
|
|
What are physiological buffers in the cells & in the blood?
|
Cells: phosphoric acid (H2PO4)
Blood: carbonic acid (H2CO3) Note: CO2 + H2O can dissociate like carbonic acid, so increased or decreased levels of CO2 (depends on breathing) can effect blood pH |
|
|
Respiratory Alkalosis
|
Blood pH > 7.45
|
|
|
Respiratory Acidosis
|
Blood pH < 7.45
|
|
|
Zwitterion
|
Formal charges on molecules, but overall charge
Ex) amino acids |
|
|
(1) What enantiomer form are most amino acids found in nature? (2) Where is the other form found?
|
1. L form
2. D form found in bacterial cell wall |
|
|
What are the 2 categories of nonstandard a.a.?
|
Modified: covalent modification of standard a.a.
Specialized: not related to standard a.a. (ex. Ornithine from urea cycle) |
|
|
Why only 20 standard amino acids?
|
Only ones found in genetic code (sequence of nucleotides: 3 nucleotides = codon)
3 stop codons - 61 codons that create a.a. 64 codons for 20 a.a. |
|
|
Why is the genetic code considered to be degenerate?
|
There can be more than 1 codon for a.a.
|
|
|
Nonessential Amino Acids
|
Can be made in the body so don't have to come from the diet
|
|
|
Essential Amino Acids
|
Can't be made in the body must come from diet
|
|
|
Are the standard amino acids the only ones found in proteins?
|
No standard amino acids can be modified post-translationally
|
|
|
What are the covalent modifications to the std a.a. ?
|
1. Phosphorylation: requires alcohol (Ser, Thr, Tyr)
2. Glycosylation: occurs in Golgi body; N-linked requires amide (ASN, GLN), O-linked requires alcohol (Ser, Thr, Tyr) 3. Alkylation: variety of a.a. |

