![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
82 Cards in this Set
- Front
- Back
|
List the enzymes of the urea cycle Which reactions requires nitrogen? |
mitochondria |
|
|
How does Malate dh reaction affect the urea cycle? |
- It is the reaction binding CAC to the urea cycle. |
|
|
Which AA are converted to alfa-ketoglutarate |
- Glutamate |
|
|
which AA are degraded to acetyl-CoA |
- Tryptophan |
|
|
which AA are degraded to pyruvate |
- Tryptophan |
|
|
Which pathways has PRPP as an intermediate? |
- Biosynthesis of Histidine |
|
|
Long term regulation of urea cycle |
Transcriptional regulation of |
|
|
Which AA are converted into oxaloaceteate? |
- Aspartate |
|
|
Which AA are converted into succinyl CoA |
- Methionine *sussen er Mett (på) Is (og) Threnger Vaselin) |
|
|
Short term regulation of urea cycle |
- Allosteric activation of Carbamoyl P synthetase by N-acetyl glutamate |
|
|
Sources of AA |
- Dietary Protein |
|
|
On which organ and organelle(s) does ira cycle occur? |
- Organ; Liver |
|
|
How is ammonia transported from Extrahepatic tissues to the liver or kidneys? |
It is transported as glutamine or Alanine |
|
|
What is transdeamination? |
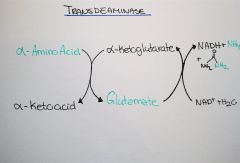
The combined action of Aminoacid transferase and glutamate dh |
|
|
Which enzyme can use both NADH and NADP? |
Glutamate dh |
|
|
What are the sources of ammonia? |
- AA degradation |
|
|
What does hyperammonia lead to? |
- Cerebral edema |
|
|
Which enzyme is responsible for elongation during protein synthesis? |
Peptidyl tranferase |
|
|
How is the termination of protein translation in bacteria and Eukaryots? |
- Bacteria: RF1 &2 recognizes stop codons and causes release of peptide transcript. RF3 (GTP binding protein) causes dissociation of RF1 & 2 - Eukaryots: e RF1 recognizes the stop codons while eRF 3 causes dissociation of eRF1 after completed polypeptide synthesis. (eRF3 is a ribosome dependent PTPase) |
|
|
Name the ubiquination enzymes and their function |
- E1; Ubiquitin activating enzyme, catalyzes the first step which targets a protein for degradation |
|
|
What is the second genetic code? |
The second genetic code is the proof reading by aminoacyl tRNA syntethase which makes sure that the amino asid added to the growing peptide chain is the right one. |
|
|
What are the respiratory chain proteins? |
In humans they are encoded in the mtDNA |
|
|
What is the start and stop codons in mitochondria and cytosol? |
Cytosol Mitochondria |
|
|
What is the differences btw prokaryotic and prokaryotic protein synthesis? |
- The size and composition of the Ribosome -Pro; 70s (50s + 30s) |
|
|
WHat are the three sites on the ribosome? |
A: Aminoacyl |
|
|
what are the different ways of post-translational protein modification? |
- Methylation |
|
|
What ensures accuracy of protein synthesis? |
- Wobble in the condon-anticodon pairing |
|
|
How are proteins moved into the ER? |
Signal recognition particles (SRP) binds to GTP, this halts the translation and directs the ribosome to the SRP receptor. SRP then binds to the peptide translocase complex in the ER membrane which moves the protein into the ER |
|
|
Properties of Mitochondrial DNA replication |
- individual mtDNA replicate randomly |
|
|
What is PLP and which reactions uses it? |
- PLP a prostetic group and the co enzyme form of vit B6 |
|
|
Part of the mitochondrial transcription |
- PolRMT = mitochondrial RNA polymerase -TFB2M |
|
|
Degradation of proteins in bacteria |
- Lon, an ATP dependent protease |
|
|
What is microautophagy? |
when small cytoplasmic vesicles are formed and endocytosed into the lysosome |
|
|
What is macroautophagy? |
entire organelles or other largecytoplasmic entieties are engulfed and then fused with the lysosome |
|
|
which molecules plays a role in protein transport into mitochondria? |
- Tom -receptor (outer memb) |
|
|
What may be caused by defect degradation of proteins? |
- Cancer |
|
|
How are proteins transported into the nucleus? |
Nuclear localization sequence (NLS) on the protein binds to importin alfa and beta thsi leads to translocation of the protein into the nucleus through a nuclear pore complex |
|
|
degradation of proteins in eucaryotes |
occurs through ATP-dependent proteolysis factor1 (APF-1 =ubiquitin) this occurs in two steps
|
|
|
What is the N-end rule? |
Average protein halg lifes correlates with the N-terminal residue |
|
|
Chaperone families with function |
-Hsp25 prevents aggregation in the lens (catararact) and protects against cellular stress' |
|
|
function of proteosome |
- endopeptidase activity (breaks peptide bonds) |
|
|
Which polymerase synthesized Eucaryotic RNA |
RNA polymerase II |
|
|
What are the true ketogenic AA? |
Leucine and lycine |
|
|
Whstis is the sequence of E.coli promoters? |
TTGACA |
|
|
Waht is the repressor binding site called? |
Operator |
|
|
What is the activator binding site called |
promotor |
|
|
What is an operon? |
an operon is a genecluster under regulation of a single promoter |
|
|
What does most antibiotics work on? |
they act against protein synthesis, usually on the ribosomre |
|
|
How can phosphorylation of initiation factors affect them? |
makes them less active |
|
|
How can hormones regulate transcription? |
- Hormonereceptor compexes binds the specific DNA sequences called hormone response elements (HRE) like dimers - can enhance or suppress transcription |
|
|
What is needed to initiate eucaryotic RNA synthesis |
- TBP -> TATA binding protein |
|
|
How does the antibioticum Tetracycline work? |
they bind to the small subunit of the ribosome |
|
|
How does the antibioticum erythromyocin work |
Blocks the polypeptide exit tunnel on the large subunit |
|
|
How does the antibioticum puromyocin work? |
it disrupts peptide bond formation by working as a aminocyl tRNA analog |
|
|
Which aminoacid is the only one who do not contain a chiral carbon |
Glycine |
|
|
mention some non polar (hydrophobic) uncharged Amino acids |
- phenylalanine |
|
|
mention some polar (hydrophilic and uncharged AA |
- glycine - glutamine |
|
|
Mention the acidic and charged AA |
Aspartate and glutamate |
|
|
Mention the basic and charged AA (Polar) |
- Histidine |
|
|
What are the properties of the peptide bond? |
- flat structure |
|
|
Peptide hormones |
- ACTH |
|
|
Name some antibiotics |
antibiotics are peptides |
|
|
Mention some neuromodulators |
neuromodulates are peptides |
|
|
mention 2 toxic peptides |
- microcystin |
|
|
wht are izozymes |
enzymes that differ in sequence but catalyze the same rxn |
|
|
what are zymogens? |
inactive procursors of enzymes
|
|
|
what are the 3 steps of protein synthesis |
1. folding |
|
|
autosomal lysosomal degradation |
- non-selective - micro and macro autophagy |
|
|
Mention some inhibitors of translation |
- Puromyocin (both) |
|
|
short term positive regulators of protein synthesis |
- IGF-1 |
|
|
Short term positive regulators of protein degradation |
- thyroid hormone excess |
|
|
Cadherin |
- Only in eukaryotes |
|
|
Integrins |
- activates signal transduction pathways |
|
|
Pathways activating HIF-1alfa |
1. RTK -> PI3K/AKT -> mTOR ->HIF-1alfa |
|
|
Which enzyme is defect in marple syrup disease? |
branched chain alfa-ketoacid dh complex |
|
|
Which enzyme is defect in argininemia? |
Arginase |
|
|
Which enzyme is defect in Phenylketuria? |
Phenylalanine hydroxylase |
|
|
Which enzyme is defect inTyrosinemia II |
Tyrosine aminotransferase |
|
|
Which enzyme is defect in tyrosinemia III |
p-hydroxyphenylpyruvate dioxygenase |
|
|
Which enzyme is defect in Tyrosinemia 1 |
Fumarylacetoacetase |
|
|
Which enzyme is defect in Alkaptonuria |
homogentisate dioxygenase |
|
|
Which enzyme is defect in Albinism |
Tyroine monoxygenase / tyrosinase |

