![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
68 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Glycine |
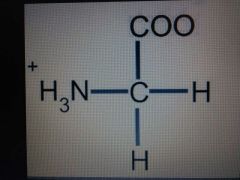
Nonpolar, Gly, G |
Only achiral amino acid |
|
|
Alanine |
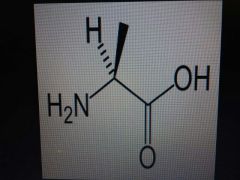
Nonpolar, Ala, A |
Methyl |
|
|
Valine |
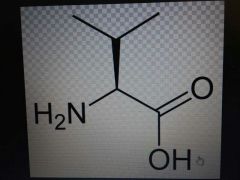
Nonpolar, Val, V |
Looks like a v |
|
|
Leucine |
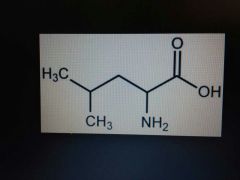
Nonpolar, Leu, L |
Similar to valine |
|
|
Isoleucine |
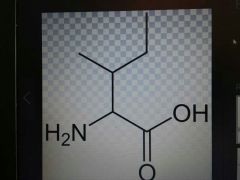
Nonpolar, Ile, I |
Leucine rearranged |
|
|
Methionine |
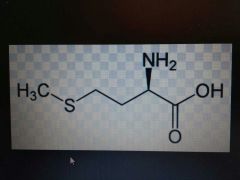
Nonpolar, Met, M |
Has sulfur |
|
|
Phenylalanine |
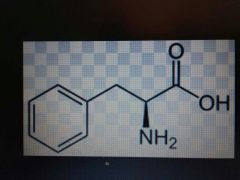
Nonpolar, Phe, F |
Phenyl group |
|
|
Tryptophan |
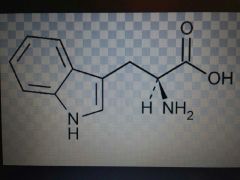
Nonpolar, Trp, W |
Trippy, 2 rings |
|
|
Proline |
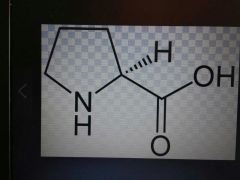
Nonpolar, Pro, P |
Amine group part of ring |
|
|
Serine |
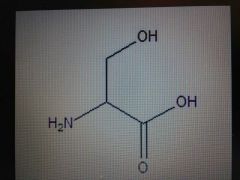
Polar, Ser, S |
Alcohol |
|
|
Threonine |
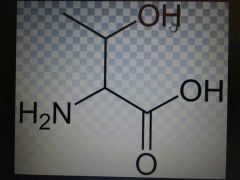
Polar, Thr, T |
Serine plus methyl |
|
|
Cysteine |
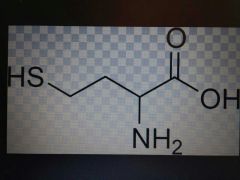
Polar, Cys, C |
Disulfide bridges |
|
|
Tyrosine |
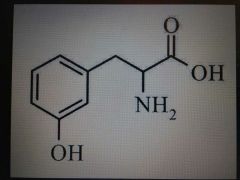
Polar, Tyr, Y |
Like F plus a little extra |
|
|
Asparagine |
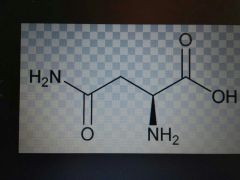
Polar, Asn, N |
Amine and ketone |
|
|
Glutamine |
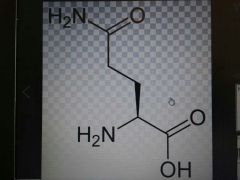
Polar, Gln, Q |
Like asn but longer |
|
|
Aspartate |
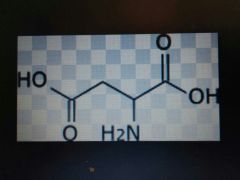
Acidic, Asp, D |
Carboxylate |
|
|
Lysine |
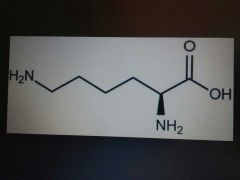
Basic, Lys, K |
4C |
|
|
Glutamte |
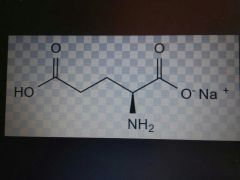
Acidic, Glu, E |
Glutamine but -ate instead of amine |
|
|
Arginine |
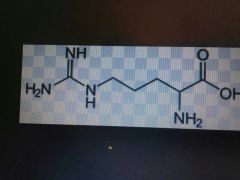
Basic, Arg, R |
3 Ns on side chain |
|
|
Histidine |
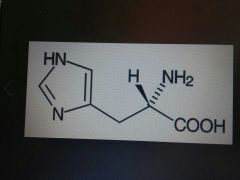
Basic, His, H |
5 membered ring with 2 Ns |
|
|
Ionization of water |
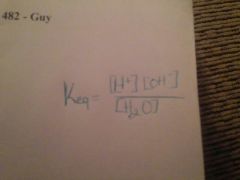
|
It's a formula you seek |
|
|
pH = |
- log [H+] |
No hint here |
|
|
Ka = |
[H+][A-]/[HA] |
Think about ionization of water |
|
|
Higher Ka = ? acid |
Stronger acid = ? Ka |
Relationship |
|
|
Lower pka (-log Ka) = ? acid |
Stronger acid = ? pka |
Relatioship |
|
|
Henderson-Hasslebach equation |
pH = pka + log [A-]/[HA] |
David Hasslehoff |
|
|
Best buffer range |
+/- 1 pH unit from pka |
Hahahaha. You thought you were gonna get a hint. :) |
|
|
Which amino acids can be phosphorylated? |
S, T, Y |
There are 3 |
|
|
Which amino acids can be hydroxylated? |
P and K |
Only 2 |
|
|
Which amino acids can be glycolsylated? |
S, T, N, Q |
4 4 4 4 4 4 4 4 4 |
|
|
Hierarchy of protein structure |
Primary<Secondary<Tertiary <Quaternary |
Primary first |
|
|
Water's amazing properties |
Polarity, solid structure allows ice to float, ionization-can act as acid or base |
3 things |
|
|
Dilution formula |
M1V1 = M1V2 |
Seriously? You want a hint? |
|
|
Titration curves show ____________ |
__________ shows the progressive dissociation of a weak acid. |
Graph |
|
|
Buffer |
Solution that resists changes in pH as acids or bases are added |
Boondoggle is a funny word |
|
|
Buffers are typically made of what 2 ingredients? |
Weak acid and conjugate base |
Nah |
|
|
What is unique about Gly? |
Only achiral amino acid |
Glycine |
|
|
Zwitterion |
Has acidic and basic properties (like amino and carboxylic acid) |
Amino acid is an example |
|
|
Peptide bond has __% double bond character. |
40% |
Less than 50 |
|
|
Oligopeptide |
12 - 20 peptides |
Large writing |
|
|
pI |
Protein has a neutral charge |
Allison rocks |
|
|
Micelle |
Lipid sphere with hydrophobic center |
Yada yada yada |
|
|
D and L refer to ______________. |
____________ refer to the configuration of a chiral molecule as compared to a standard (glyceraldehyde) |
Sbubisbinoma o nebi as non |
|
|
Approximate pka of carboxyl group |
pka ~ 2 |
=-O |
|
|
Approximate pka of amino group |
pka ~ 9 |
(:V) |
|
|
Which amino acids can be seen under UV light? |
Phe, Trp, & Tyr |
3 3 3 3 3 3 3 3 3 3 |
|
|
Ways to separate proteins (6) |
Solubility, size, shape, charge, amino acid sequencing, ligand binding |
s,s,s,c,a,l |
|
|
Dialysis |
Separates proteins by size while maintaining native state |
Not the kidney kind |
|
|
Gel filtration chromatography |
Smaller proteins go inside gel beads and move through column slower than larger proteins |
Size separation |
|
|
SDS-PAGE |
Gel electrophoresis. Detergent denatures proteins |
I'm done with hints. Sorry |
|
|
Ion exchange |
Separates proteins by charge (anion and cation) |
@@@@@@@@@ |
|
|
Ways to detect protein with color change |
Coommassie brilliant blue and BCA - copper reduction causes punk color |
Blue & pink |
|
|
Common protein tags |
His6, FLAG, HA, myc |
I like chocolate |
|
|
Crude extract |
Complex mixture of proteins and other molecules after cell lysis and centerfuging |
Blow up some cells. Woo hoo |
|
|
Specific activity |
Total activity/total protein |
Units/mL |
|
|
Yield |
Activity @ that step/activity of crude extract |
Sooooooo tired |
|
|
Purification level |
Specific activity@ that step/specific activity of crude extract |
BOLD |
|
|
Step 1 of sequencing a.a.:separate chains |
extreme pH, urea, guanidine, high salt |
4 ways |
|
|
Step 2 of sequencing a.a.: cleave disulfide bridges |
BME or DTT then iodacetate to keep from reforming |
Reducing agent + alkylating agent |
|
|
Step 3 of sequencing a.a.: N- and C- terminals |
N: Edman degredation C: carboxypeptidase (a-all except pro, arg, lys; b-arg and lys) |
Different for n and c |
|
|
Step 4 of sequencing a.a.: fragment the chains |
Done multiple time. (Trypsin, chymotrypsin, clostripain, staphylococcal protease, cyanogen bromide) |
5 kinds |
|
|
Step 6 of sequencing a.a.: reconstruct the sequence |
Overlap sets of fragments |
Mass spec can make fragments too |
|
|
Trypsin |
Arg or Lys |
C side |
|
|
Chymotrypsin |
Phe, Trp, Tyr, Leu |
C side again |
|
|
Clostripain |
Arg |
C side still |
|
|
Staphylococcal protease |
Asp or Glu |
Sea side |
|
|
Cyanogen bromide |
Met |
Always c side |
|
|
Mass spec advantages/disadvantages sequencing proteins |
Good: can ID mix of prpteins; fast and little material needed Bad: need some knowledge of genome; doesn't always work |
:-) :-( :-P ;-) =-O :-[ :-\ :'( :-D :-! :-$ :-)) :-| |

