![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
PKA's for Asparate, Gltuatmate, Histidine, Cystine, Tyrosine, Lysine, Arginine |
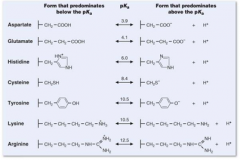
|
|
|
Name 6 major enzyme classifications and what they do |
oxidoreductases - Redox reaction transferases - Move group hydrolases - Cut with water lyases - Cut without using H2O or redox isomerases - Isomerize ligases - Combine |
|
|
How does enzyme act as catalyst |
Stabalizing normal transition state or providing different pathway |
|
|
Ways that transition states stabalize transitions states: |
Induced Fit Acid Base catalysis Covalent Catalysis Metal Ion catalysis |
|
|
Cofactors of: Activation transfer vs Redox |
Activation transfer use the transfering group as cofactor Redox transfers electrons and does not from covalent bonds |
|
|
Describe mechanism for serine Proteases |
Stabilizes intermediate through H bonding and covalent bonding. Holds Reactants where they need to be in order to be "attached" |
|
|
Explain Michaelis-Menton Equation Kinetics |
Vi=(Vmax [S])/[Km+S] Initial speed = how fast it is 50% saturated Km= substrate affinity higher is greater affinity *also the substrate concentration needed to reach 1/2 Vmax Vmax= Max velocity at infinite concentration - Reaction won't go any faster |
|
|
Compare Glucokinase vs Hexokinase with respect to their Km's |
Hexo: Low Km = fast uptake Gluco: Higher= slower uptake |
|
|
How does Km and Vmax change with Comp vs noncomp inhibitors |
Competetive: Km higher Vmax the same Non Comp: Km SAME Vmax lower |
|
|
How does enzyme concentration change with rates of transcription or degradation? |
More transcription= more enzymes
More degradation = less enzymes |
|
|
Alosterism and activation vs inhibition |
Activation binds to keep it relaxed Inhibition binds to keep it tense |
|
|
R vs T in enzyme bindong |
R=Relaxed and able to do work T= Tense and not able to work |
|
|
Protenolysis |
Changing of Zymogens to heir active state by removing part of the protien |
|
|
Reversale Phosphorilization |
Adding or removing a Phosphorylate group to activate to deactivate a protein |
|
|
How does substrate concentration affect Vi? |
Higher S, higher Vi. Vmax times S and Km PLUS S |
|
|
Thiamins phosporilase |
Cofactor |
|
|
Biotin |
Water soluble B-Vitamin used as coenzyme |
|
|
Pyrodoxal Phospate |
Coenzyme that reacts with amine group of amino acid |
|
|
Covalent Modificaitons |
Phyosphoralization of Serine, Tyrosine and sometimes threonine Changes activity of protein Can be dephosphoralized and return to previous state. |
|
|
Protien protien interactions |
When one protein interacts with another protein. GPCR for Ex.
|

