![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
Glucose Oxidation
|
C6H12O6+6O2----->6CO2+6H2O
|
|
|
NADH and FADH2 produced from glycolysis and TCA
|
10 NADH and 2 FADH2
|
|
|
Chemical Coupling Hypothesis
|
ATP produced via substrate level phosphorylation of ADP by reactive intermediates (X~P).
|
|
|
Chemoiosmotic hypothesis
|
use of an electrochemical gradient or proton-motive force to drive ATP synthesis.
|
|
|
If the “__________” flow of electrons from NADH to oxygen occurs in a stepwise manner, then some of this energy can be harnessed to
“pump” protons and create an electro- chemical potential. |
downhill
|
|
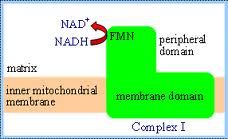
What does this represent?
|
Complex 1
|
|
|
Characteristics of Complex I
|
-largest e- transport protein
-redox centers are prosthetic groups -e-'s tunnel through covalent bonds -as e-'s are transferred from NADH to ubiquinone, complex I transfers 4 protons from matrix to the inter membrane space. -proton "wire" serves as the relay mechanism |
|
|
Reaction of Complex I
|
NADH+H+Q---->NAD+QH2
ubiquinone oxioreductase or NADH dehydrogenase |
|
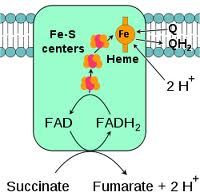
Characteristics of Complex II
|
succinate+Q-->fumarate+QH2 by succ DH
<--- -not direct contribution of ATP synthesis -bypasses complex 1 |
|
Complex III Characteristics
|
-transfers from Q to cytochrome C
-resonance stabilized Q radical is not as reactive -cytochrome=proteins with heme prosthetic groups -heme undergoes one e- reduction with central Fe atom cycling b/t Fe3+ and Fe2+ (reduced) aka uses heme group for e- transfer -"Hands off" electrons -give two protons to intermembrane space |
|
|
Reaction of Complex III
|
QH2+2cytochromeC[Fe3+]--->
<---Q+2cytochromeC[Fe2+]+2H+ |
|
|
Two Round Q Cycle
|
-2 e-'s from QH2 reduce 2 molecules of cytochrome C
-4 protons are translocated to the intermembrane space, two from QH2 in first round and two from QH2 in second round -end of round two: QH2 regenerated |
|
|
Cytochrome IV
|
-oxidizes cytochrome C and reduce O2 (consumes 4 protons in mitochrondiral matrix)
4 cytochromeC[Fe2+]+O2+4H+---> <----4cytochromeC[Fe3+]+2H2O -redox centers=heme groups and copper ions -water and proton relays deplete the matrix H+ gradient to form H+ gradient across inner mit membrane. -copper-sulfur |
|
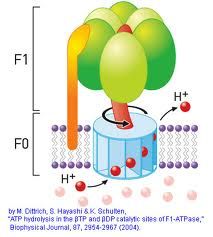
Characteristics of ATP Synthase
|
-proton translocation drives a portion of ATP synthase
-supply of reduced cofactors determines rate of oxidative phosphorylation -3 aB pairs change conformations as the gamma subunit rotates -rotation-driven conformational changes alter affinity of each catalytic B subunit -greatly favored process thermodynamically (low activation barriers) -Takes protons from intermembrane to matrix side |
|
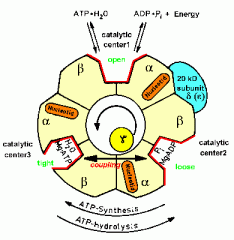
Steps of ATP synthase
|
1. ADP+Pi bind to B subunit loosely
2. Converted to ATP; gamma subunit rotation--->B subunit shift to tight conformation 3. B subunit shifts to open conformation |
|
|
In absence of H+ gradient, is ATP synthesized?
|
No, because no free energy to drive gamma subunit rotation
|
|
|
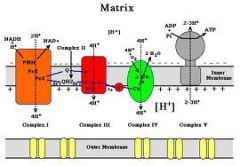
Pathways of Oxidative Phosphorylation
|
I--->Q--->III or II-->Q--->III
|

