![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
86 Cards in this Set
- Front
- Back
|
Hemoglobin Evolution - atmospheric considerations
|
atmosphere changed from reducing to oxidizing 2 billion years ago
Fe+2 oxidized to Fe+3 - mass extinction electron-deficient properties of O2 harnesed to increase oxidative metabolism efficiency and diversity |
|
|
Globin secondary structure
|
alpha-helices (78%), remainder in loops joining the helices
no beta sheets |
|
|
Hemoglobin Evolution - phylogeny
|
single globin chain -> two chains via gene duplication (alpha and beta) during higher fish evolution - marine worms, insects, primitive fish have single globin chain
alpha and bega genes translocated to different chromosomes in birds and mammals, but on same chromosome in frog Xenopus in mammals alpha and beta chains duplicated and diversified |
|
|
Hemoglobin α and β chains in mammals
|
beta chain has greatest effect - beta chains have different 02 binding affinities.
beta diversified into fetal (ε,γ) and adult (β,δ) forms. tetramers of 2α2β required for functionality - β homotetramer possible if β overexpressed but it is not functional fetal beta forms (epsilon and gamma) have higher 02 affinity, allowing transfer from maternal to fetal complexes. alpha2delta2 heterotetramer a minor component of primate adult hemoglobin |
|
|
Ligand
|
molecule that reversibly binds to an protein. Removal of water from the binding site and the ligand (desolvation) results in an initial activation energy cost.
ligand and protein have binding site complementarity which equates to high specificity |
|
|
Ligand binding
|
concentration-dependent, high concentration increases likelihood of collision between specific protein and ligand allowing maximum binding interactions
electrostatic charge complementarity can also direct binding. binding coupled to conformational change in protein |
|
|
dissociation constant
|
Kd = ([P][L]) / [PL]
P=protein, L=ligand Smaller Kd => greater affinity Kd is [L] at which 50% of binding sites are occupied |
|
|
θ
|
fraction of binding sites occupied by ligand
θ = (binding sites occupied)/(total binding sites) θ = [PL] / (]PL] - [P]) θ = [L] / ([L] + Kd) [L] = (θ Kd) / (1 - θ) |
|
|
biological O2 binding
|
Reduced transition metals can bind O2 - Fe2+ and Cu+, but not Fe3+ and Cu2+
Fe2+ spontaneously oxidizes with O2 and hydroxyl radicals are formed four N form porphyrin coordinate with Fe2+ and stabilize the 2+ state ofer the 3+ 2 free hemes + O2 = irreversible oxidation to Fe3+ Heme sequestered in protein with His (F8) coordinated to one of the two remaining sites - stabilizes the Fe2+ by donating e- and leaves only one binding site for O2 O2 binds reversibly to heme so sequestered and there is no change in Fe oxidation state |
|
|
Myoglobin (Mb)
|
single chain globin
most abundant in muscle, aids O2 transport through tissue Diving mammals have high Mb in muscles which serves as an O2 store also. |
|
|
Myoglobin and O2 binding
|
partial pressure of O2 measured (dissolved O2 is difficult to measure)
θ = pO2 / (pO2 + P50) P50 = partial pressure of O2 at which half of ligand sites are occupied |
|
|
CO
|
binds free heme with 20000 X higher affinity than O2
heme bound to Mb or Hb CO affinity 200 (Mb) or 250 (Hb) X greater than O2 - CO cannot form linear conformation due to steric hinderance due to a His residue The His residue stabilizes the bent O2 binding |
|
|
CO poisoning
|
more than 50% of worldwide poisoning deaths
CO bound to 1 or 2 Hb subunits increases O2 affinity at other sites - does not release O2 at the tissues |
|
|
Siclke cell anemia
|
point mutation - Glu to Val - homozygotes show symptoms
beta chain conformation not significantly changed hydrophobic residue placed on surface The Val fits into hydrophobic pocket of another Hb when deoxygenated deoxyHbS polymerizes into fibers during anoxic conditions - physical exertion Hb content of sickle-cell blood is ~50% that of normal individuals - sickle cells are fragile and rupture easily sickle cells can block small capillaries heterozygotes have slight resistance to malaria |
|
|
Hemoglobin structure
|
4 subunits (2 alpha, 2 beta)
subunits have globin tertiary fold with a heme prosthetic group - each Hb can bind up to 4 O2 alpha and beta subunits are adjacent and have more interactions than between alpha-alpha or beta-beta which are opposite |
|
|
Hemoglobin states
|
T state - more stable in absence of O2,
R state - higher O2 affinity, O2 binding stabilizes R state Transition does not cause large change in individual subunits, but extensive changes at the alpha-beta interfaces - T -> R disrupts some ionic interactions, while some new ones are formed. α-β interface has >30 interactions, most hydrophobic, and some ionic (salt bridges or ion pairs) |
|
|
inactive forms of enzymes
|
proenzymes or zymogens
common for proteases, e.g. chymotrypsin from chymotrypsinogen by cleavage of short N-terminus peptide upon entry into digestive tract |
|
|
proteases
|
degrade proteins
inactivated by inhibitor proteins that are tightly bound to the active site e.g. pancreatic trypsin inhibitor |
|
|
proteosome
|
in eukaryotes, degrades proteins
constitutes about 1% of cellular protein consists of 20S core and two 19S caps some core subunits are proteases with active sites lining cylinder 19S caps composed of ca. 18 subunits, ~6 of which hydrolyze ATP proteins targeted for transport to proteasome by attachment of chains of ubiquitin |
|
|
ubiquitin
|
76 a.a. protein
C-terminus covalently linked to amino group on side chain of K (isopeptidic bond) Chains of at least 4 ubiquitins are needed to target proteins to the proteasome C-terminus also attaches to K of another ubiquitin to form chains |
|
|
ubiquitination - associated enzymes
|
E1 - ubiquitin-activating enzyme
E2 - ubiquitin-conjugating enzyme (~30 in mammals) E3 - ubiquitin ligase (>200 in mammals) E2 and E3 act as a complex E3 is key, recognizes different degradation signals on proteins ca. 300 E2/E3 combinations found in mammals |
|
|
ubiquitin activation
|
E1 forms high-energy thioester bond between a C residue and the C-terminus of ubiquitin via an ATP coupled reaction.
E1 then transfers ubiquitin to a C residue on the E2 of an activated E2/E3 complex |
|
|
ubiquitination - targeting
|
E3 recognizes specific degradation signals.
This allows E2 to transfer ubiquitin to a K residue on the target The proteosome 19S cap recognizes the polyubiquitin chain. Ubiquitin is cleaved from target protein and re-used |
|
|
ubiquitination - ligase complex activation
|
E2/E3 complex activated by:
-phosphorylation -allosteric transition due to ligand binding -allosteric transition due to protein subunit addition These processes can also occur on the target protein which helps mark it for ubiquitination |
|
|
ubiquitination - N-terminal targeting
|
the N-terminal residue can target protein for degradation - identity correlated to half-life in the cell in a clock-like manner
Stabilizing residues are: M, G, A, S, T, V. these give a half-life of >20 hours. Destabilizing residues (some others others) give half-lives from 2 to 30 minutes |
|
|
Metabolism - general
|
catabolism - breakdown of energy-containing compounds. Energy stored in high-energy or reduced compounds, e.g. ATP and NADH
anabolism - synthesis of complex molecules - requires free energy catabolism converges on cyclic pathway, while anabolism diverges to create wide variety of compounds |
|
|
metabolism - categories of reactions
|
4 major categories:
1. Oxidation/reductio 2. Reactions that make or break C-C bonds. 3. Internal rearrangements (isomerizations like trans to cis P) and eliminations (e.g. dehydration) 4. Group transfers: acyl, glycosyl, phosphoryl, etc. |
|
|
Group transfer
|
via a Sn2 mechanism with tetrahedral intermediate
Phosphoryl transfer - from ATP is the main way chemical energy is transferred during catalysis |
|
|
ATP Hydrolysis - large negative free energy change
|
1. Relief of electrostatic repulsion between the 4 negative charges by separation
2. Pi is resonance stabilized. 3. ADP(2-) immediately ionizes releasing H+ at ~ph =7 4. Greater degree of solvation relative to ATP |
|
|
non-ATP chemical energy stores
|
PEP3- to pyruvate- and Pi
Acetyl-CoA to acetic acid to acetate ion Phosphocreatine to creatine |
|
|
ATP as energy currency
|
ATP is intermediate in phosphorylation potential - it can transfer phosphoryl groups from higher-energy phosphate compounds from catalysis to other compounds, such as glucose
ATP is kinetically stable - does not spontaneously transfer Pi groups, requires enzyme |
|
|
ATP group transfer mechanism
|
usually written as 1-step hydrolysis, but generally achieved by 2-step covalent group transfer
processes that involve direct hydrolysis of ATP usually linked to mechanical motion e.g. protein comformational changes, muscle contractions, movement of protein complexes along DNA |
|
|
ATP structure
|
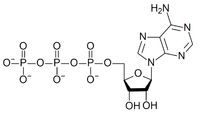
|
|
|
nucleophilic attack on ATP
|
3 positions for attack:
γ - phosphoryl transfer; R-Pi + ADP β - pyrophosphoryl transfer; R-P-Pi + AMP α - adenylyl transfer; R-P-Adn + PPi pyrophosphoryl transfer releases greater G than phosphoryl - used for more unfavorable reactions e.g. ubiquitin charging |
|
|
adenylyl group transfer
|
adenylyl group = 5'AMP
generates pyrophosphate (PPi) which is further hydrolyzed to release more free energy by inorganic pyrophosphatase coupled to very unfavorable reactions such as fatty acid activation |
|
|
fatty acid activation
|
highly unfavorable, driven by adenylylation
adenylyl transferd to carboxyl group of fatty acid, then coenzyme A displaces the adenylyl group to form thioester |
|
|
metabolic regulation
|
12% of human genes encode regulatory proteins
maintaining constant [ATP] is second most important task for cells typical [ATP] = 5mM in cells typical ATP-consuming enzymes have Km ~ .0 to 1 mM if [ATP] decreases, many reactions would not occur and cell would die [ATP]/[ADP] is critical |
|
|
[AMP] as indicator
|
[AMP] usually < 0.1mM
[ADP] usually ~ 1.0mM Adenylate kinase catalyzes: 2ADP -> AMP + ATP drop in [ATP] leads to greater relative increase in [AMP] than [ADP] many reactions are regulated by [AMP] |
|
|
induced fit
|
protein conformational changes upon initial binding with ligand to optimize interactions
|
|
|
O2 binding to Hemoglobin
|
-can bind to both states, but R has higher affinity.
-binds in the distal pocket, which stabilizes the R-state T-state has porphyrin ring slightly bent toward the proximal His ligand - on binding O2, porphyrin ring becomes more planar |
|
|
proximal and distal pockets
|
proximal - binding pocket has porphyrin ring bowed toward the proximal His. Occurs in the T state.
distal - pocket more open, porphyrin ring more planar, O2 (or other ligand/solvent) can bind. |
|
|
Hemoglobin cooperative binding
|
transition from low to high affinity states.
Transition from T to R state is positively cooperative - each O2 that binds, raises affinity in the remaining subunits. produces sigmoidal curve on a plot of θ vs. pO2. (non-cooperative binding produces hyperbolic curve) |
|
|
Allostery
|
binding of a ligand to one site causes a conformational change that alters affinity at another site.
positive and negative cooperativity heterotropic - different ligands at each site homotropic - same ligands bind at each site |
|
|
allostery models
|
symmetry model - all sites either in high or low affinity states. Ligand binding increases high affinity state and alters equilibrium between states. All or nothing change in quaternary structure.
sequential model - individual sites can be in different states. Ligand binding increases proportion of sites in high-affinity state. |
|
|
cooperative binding equations
|
for n-sites:
Kd = ([P][L]^n) / [PL] θ = ([L]^n) / ([L]^n = Kd) |
|
|
Hill equation
|
calculates the relative saturation
θ / (1 - θ) = ([L]^n) / Kd |
|
|
Hill plots
|
log(θ / (1 - θ)) vs. log[L]
[L] = conc. of free L slope = n |
|
|
Hill coefficient
|
nsubh
measure of degree of cooperativity n ≠ # of binding sites * n is always less than # binding sites * |
|
|
hemoglobin and myoglobin Hill plots
|
myoglobin n = 1
hemoglobin T and R states n=1 hemoglobin in cooperative range n ≈ 3 hemoglobin - linear with slope = 1 myoglobin - sigmoidal with two asymptotes |
|
|
Bohr effect - hemoglobin in tissues
|
CO2 from metabolism converted to carbonic acid (H2CO3) by anhydrase - then dissociates to lower pH. Tissue pH ≈ 7.2.
This protonates some of the residues involved in ionic interactions between the subunits which favors O2 release A His residue on the β subunit forms ionic pair with an Asp on the α subunit. This gives the His an abnormally high pKa and the ion pair stabilizes the T state. O2 released into the tissues, CO2 (carbonic acid) taken into erythrocytes. Proton binding antagonistic to O2 binding |
|
|
Bohr effect - hemoglobin in lungs
|
lung pH = 7.6. The R-state does not have this ion pair and the His pKa drops back to 6 and is mostly unprotonated.
High pO2 in lungs favors O2 binding, stabilizing the R-state. Released protons combine with bicarbonate to form CO2 via carbonic anhydrase. CO2 exhaled O2 binding antagonistic to CO2 (proton) binding. |
|
|
BPG
|
2,3-bisphosphoglycerate
formed in erythrocytes heterotropic allosteric modulator of Hb |
|
|
BPG and Hb
|
single BPG binds to positively charged cavity between subunits in T-state. This makes the transition to R more difficult and lowers O2 affinity.
little effect on O2 bound in the lungs, large effect on release in the tissues. maintains ≈ 40% O2 delivery to tissues at different altitudes (different pO2) without PBG Hb's high O2 affinity would prevent release in the tissues Fetal Hb (HbF) has lower BPG affinity and thus higher O2 affinity than adult Hb (HbA). This allows O2 transfer from maternal to fetal Hb across the placenta. |
|
|
catalyst
|
alters rate (but not equilibrium) of a reaction by many orders of magnitude
following a complete cycle, original state is returned |
|
|
catalytic power
|
rate of catalyzed reaction over rate of uncatalyzed reaction
for enzymes usually 10e5 to 10e17 |
|
|
6 classes of enzymes
|
1. oxidoreductases - xfer of electrons (hydride ions or H atoms)
2. transferases - group transfer 3. hydrolases - hydrolysis reactions (transfer of groups to water) 4. lyases - addition of groups to double bonds / formation of double bonds by removal of groups 5. isomerases - internal rearrangements 6. ligases - formation of C-C, C-O, C-S, and C-N bonds by condensations coupled with ATP cleavage |
|
|
enzymes - general
|
most are proteins, few are RNA (ribozymes)
some require cofactors such as metal ions some require coenzymes such as NAD+ or FAD prosthetic groups are coenzymes or cofactors that are covalent bonded to enzyme holoenzyme = cofactor/coenzyme + apoenzyme/apoprotein complementarity of binding sites gives high specificity - usually stereospecific no effect on ΔG or Keq |
|
|
Keq and free energy
|
10fold increase of K'eq ≈ 6 kJ/mol of stabiliztion or addition of ca. 1.5 hydrogen bonds per molecule
|
|
|
enzymes and equilibria
|
enzymes speed up both forward and backward reactions equally (think of carbonic anhydrase in the tissues vs the lungs)
overall product/reactant ratio of multiple sequential reactions influenced by individual Keq - coupling an unfavorable to a favorable reaction can result in product accumulation |
|
|
enzyme kinetics
|
various distinct reaction intermediates formed with enzyme
most strained structure between intermediates is the transition state- decay into P or S equally likely at this point enzymes lower energy barrier enzymes stabilize particular transition states |
|
|
reaction intermediate
|
has a finite lifetime - longer than a molecular vibration
|
|
|
transition state
|
transient, not possible to isolate
|
|
|
reaction rates
|
determined by concentration of reactants and rate constant k
unimolecular reaction: V = k[S] ; k is first order with units of s-1 bimolecular reaction: V = k[S][Y] ; k is second-order with units of M-1s-1 k = (kT/h)e^(-ΔG‡/RT) |
|
|
ΔG‡
|
transition state energy barrier
contributing factors: 1. reduction of entropy required to orient 2 molecules correctly 2. removal of solvation shell from reactants 3. distortion or strain during reactions 4. proper alignment of catalytic groups |
|
|
Michaelis-Mente Equation
|
V0 = kcat[ES]
|
|
|
Km
|
Michaelis constant
=[S] at which V0 = 0.5 Vmax |
|
|
Lineweaver-Burk plot
|
double-reciprocal plot
1/Vmax = y-intercept -1/Km = x-intercept |
|
|
bisubstrate reactions
|
sequential and ping-pong
2 substrates going to two products, most common are transferase reactions |
|
|
sequential reaction
|
both substrates bind to form ternary complex.
Double reciprocal plot with several lines for different [S] - lines intersect. |
|
|
ping-pong reaction
|
double-replacement
no ternary complex formed substrate 1 binds and leaves as product 2 - modifies enzyme substrate 2 binds and leaves as product 2 - restores enzyme Double reciprocal plot with lines for different [S] - lines parallel |
|
|
inhibition
|
inhibit catalytic activity through binding
competitive or non competitive |
|
|
competitive inhibition
|
know equation
lines intersect in double reciprocal plot at the y-intercept (1/Vmax) - changes Km, but not Vmax competitive inhibitor binds to same active site - best are analogs of the transition state |
|
|
uncompetitive inhibition
|
binds to the ES complex
often in multisubstrate reactions - often resembles second substrate (competitive with respect to second substrate) lines parallel on double-reciprocal plot - decreases Vmax and Km |
|
|
mixed inhibition
|
binds away from the substrate binding site
can bind to both free enzyme and complex can alter binding affinity for substrate |
|
|
noncompetetive inhibition
|
special case of mixed
does not alter substrate affinity, but prevents the reaction from occuring double-reciprocal - lines intersect at x-axis - Km not affected |
|
|
reversible inhibition
|
know equation
simplifies to competitive and uncompetitive equations when α' and α = 1 |
|
|
irreversible inhibition
|
covalent binding
suicide inhibition - undergoes first setps of enzymatic reaction, but instead of forming product, forms very reactive compound that binds to enzyme covalently. |
|
|
general acid-base catalysis
|
transfer of proton (to group other than water) to stabilize intermediates
|
|
|
specific acid-base catalysis
|
transfer of proton to water to stabilize interiediates
|
|
|
covalent catalysis
|
catalyst has nucleophilic group
|
|
|
metal ion catalysis
|
-can help to orient substrate
-can participate in redox through changes in oxidation state -can stabilize charged intermediates or transition states |
|
|
serine proteases
|
catalyzes polypepptide backbone - yields smaller polypeptides
-have unusually reactive seine residue - all use same mechanism trypsin, chumotrypsin, elastase (divergent evolution) other families by convergent evolution -also esterases (in addition to amidases) |
|
|
esterase activity of serine proteases
|
burst phase - formation of acyl-enzyme intermediate
steady state phase - slower hydrolysis of acyl-enzyme intermediate ping-pong mechanisom |
|
|
catalytic residues of chymotrypsin
|
Ser195, His57, Asp102 - catalytic triad
|
|
|
peptidoglycan
|
NAG-NAM disaccharide repeat linked to tetrapeptide
tetrapeptide has 2 L-a.as both saccharides are pyranoses (6-membered rings) NAM has lactyl group on C3 |
|
|
HEWL
|
hen egg white lysozyme
NAM can only fit in sites B,D,F cuts between D and E D must be in half-chair conformation |

