![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
240 Cards in this Set
- Front
- Back
|
Polypeptides are linear _______.
|
heteropolymers
|
|
|
Proteins are folded into specific shapes based on 1°, 2°,3°,and 4°. These shapes are called the ______ of the protein.
|
native conformation
|
|
|
Molecular weight of proteins is highly variable and is expressed in ______.
|
kilodalton (kDa)
|
|
|
Proteins function as...
|
1. Enzymes
2. Regulators of gene expression 3. Hormones, growth factors, receptors, signal transducers 4. Structural elements 5. Antibodies and other elements of the immune system |
|
|
In the case of starvation, proteins can function as _______.
|
an energy source
|
|
|
Proteins can be infectious particles in the form of _____.
|
prions
|
|
|
A simple protein contains only ______. A conjugated protein contains ______.
|
amino acids; non-protein components, including lipids, carbohydrates, nucleic acids, metals, and organic prosthetic groups.
|
|
|
Proteins are polymers of ____.
|
Alpha amino acids.
|
|
|
Alpha amino acids can have two isomeric structures (enantiomers), named _____.
|
L and D
|
|
|
Are proteins made of L or D amino acids?
|
L
|
|
|
D amino acids are rare and can be found in bacteria ______, toxins from ______, or as ________ in mammals.
|
peptidoglycans, cone snails and platypuses, neurotransmitters
|
|
|
As pH changes, so does the ________ of the amino acid.
|
ionization state
|
|
|
When the amino acid has both a positive and negative charge, and has no net charge, it is referred to as a _______.
|
Zwitterion
|
|
|
When pH=pK, that particular site is ____ ionized. When pH is near the pK of a weak acid or base, the weak acid or base can act as a _____.
|
50%, buffer
|
|
|
The pH where the aa has no net charge is referred to as the ______.
|
isoelectric point or pI. This will happen in between the pK of the amino group and the pK of the carboxyl group unless the R chain has an ionizable group.
|
|
|
How many L-alpha-amino acids are found in proteins?
|
20
|
|
|
Name the amino acids with positively charged side chains.
|
Lysine(K), Arginine(R), Hystidine(H, also aromatic)
|
|
|
Name the amino acids with polar, uncharged side chains.
|
Serine(S), Threonine(T), Cysteine(C), Asparagine(N), Glutamine(Q)
|
|
|
Name the amino acids with nonpolar aliphatic side chains.
|
Glycine(G), Alanine(A), Valine(V), Leucine(L), Isoleucine(I), Proline(P), Methionine(M)
|
|
|
Name the amino acids with negatively charged side chains.
|
Aspartate(D), Glutamate(E)
|
|
|
Name the amino acids with aromatic side chains.
|
Histidine(H, also can be positively charged), Phenylalanine(F), Tryosine(Y), Tryptophan(W)
|
|
|
A mutation of an amino acid to an amino acid with similar properties is called a ______ and is more likely to be tolerated in a protein.
|
conservative mutation
|
|
|
______ amino acids can form salt bridges.
|
charged
|
|
|
During the titration of aspartic acid, you will see three ______. The pI is midway between the pKs of _____ and _____.
|
buffer zones; the carboxyl group; the side chain
|
|
|
During the titration of lysine, there will be three ______. The pI will be midway between the pKs of____ and _____.
|
buffer zones; the side chain; the amino group
|
|
|
At physiological pH, glutamic acid will be ______. At physiological pH, asparagine will be ______.
|
negatively charged; positively charged
|
|
|
____, ____, and ____ will absorb light at ______. This gives them all a unique __________.
|
Tryptophan; tyrosine; phenylalanine; extinction coefficient.
|
|
|
______ has only one _____, and is the least frequent, and largest of the amino acids.
|
Tryptophan; codon
|
|
|
______ is the only nonchiral amino acid.
|
Glycine
|
|
|
The polar, noncharged amino acids contain _____, _____, and _____ groups.
|
hyrdoxyl; sulfhydryl; primary amide
|
|
|
______ is involved in disulfide bond formation.
|
Cysteine
|
|
|
L-DOPA and 5-HTP are both examples of proteins functioning as ______. Briefly describe what L-DOPA and 5-HTP do.
|
neurotransmitters; L-DOPA is the precursor of dopamine, epinephrine, and norepinephrine and is used to treat Parkinson's. 5-HTP is the precursor of serotonin.
|
|
|
9 of the amino acids are essential. Name them. What is the mnemonic device we were given in class?
|
1. Ingesting (Isoleucine)
2. These (Threonine) 3. Little (Leucine) 4. Molecules (Methionine) 5. Has (Histidine) 6. Value (Valine) 7. When (Tryptophan) 8. Knowledge (Lysine) 9. Fails (Phenylalanine) |
|
|
Animal proteins are termed "complete" because they contain ______.
|
all the essential amino acids
|
|
|
Name two diseases that belong to the class known as Protein-Energy Malnutrition (PEM).
|
marasmus and kwashiorkor
|
|
|
_________ happens when the body is unable to convert ______ into tyrosine. ______ and its breakdown products build up in the blood, causing _________.
|
Phenylketonuria (PKU), phenylalanine, phenylalanine, mental retardation
|
|
|
Individuals with phenylketonuria (PKU) must avoid the sweetener ________, which is an ester of the ________ dipeptide.
|
aspartame; Asp-Phe
|
|
|
The position of the peptide bond can be described using two rotation angles _____ and _____. The angles are relative to the ______ plane.
|
phi; psi; H-Calpha-Rgroup
|
|
|
_____ is the N-Calpha angle. _____is the Calpha-C angle
|
Phi; psi
|
|
|
In some cases, protein folding is aided by helper proteins called _______.
|
chaperones
|
|
|
Name some secondary structures of proteins.
|
Alpha helix, beta sheet, beta strand, beta turn, random coil.
|
|
|
What is the Ramachandran plot?
|
It shows the favored angles of psi and phi. The graph shows that there are two places where most of the combinations will fall. One represents the angles of the alpha helix (smaller area on the bottom), the other represents the angles of the beta sheet (larger area on the top).
|
|
|
The alpha helix is a ____ handed coil with _____ residues per turn and a pitch of ____ angstroms. The helix is held together by ________ between the ______ atoms.
|
right; 3.6; 5.4; hydrogen bonds; backbone
|
|
|
What is an amphipathic helix? Where might you find one?
|
This is when all the hydrophic aas line up on one side of an alpha helix, with all the hydrophilic aas on the other side of the helix. You might find this in a membrane protein
|
|
|
Alpha helices in transmembrane proteins will usually be one of these two types.
|
1. A single hydrophobic helix
2. a barrel of amphipathic helices (often 7), with their hyrophilic sides on the inside |
|
|
What is a leucine zipper?
|
Two alpha helices that are "zipped" together by leucine that is repeated every 7 residues (roughly two turns) of each helix.
|
|
|
Which secondary structure is most common? Second most common?
|
1. alpha helix
2. beta sheet |
|
|
In beta sheets, the backbones are almost ______. The sheet is held together by ________ between the backones.
|
fully extended; hydrogen bonds
|
|
|
Porins are _______ proteins that are made entirely of _______. They are much less common than ______.
|
transmembrane; beta sheets; alpha helices
|
|
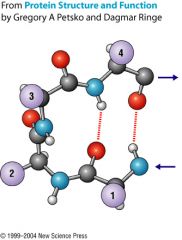
What protein structure is represented? Which two amino acids are often found in this kind of turn?
|
Beta turn. Proline and glycine.
|
|
|
Beta turns often promote the formation of ________.
|
antiparallel beta sheets
|
|
|
What is supersecondary structure? Name three examples.
|
The aggregate of adjacent secondary structures to form familiar motifs, such as the beta-alpha-beta, the beta-meander, and the Greek key.
|
|
|
Supersecondary structures: The _____ is common in DNA binding proteins. The ____ is common in calcium binding proteins.
|
helix-loop-helix, E-F hand
|
|
|
Protein disulfide isomerase...
|
helps proteins find the correct disulfide bond.
|
|
|
Prosthetic groups are...
|
non polypeptide structure or inorganic ion tightly bound to a protein and essential for its function.
e.g. heme in hemoglobin |
|
|
snRNP stands for....
|
small nuclear ribonucleoproteins
|
|
|
______ are RNA-protein complexes that combine with unmodified pre-mRNA to form a _____.
|
snRNPs; spliceosome
|
|
|
The splicing of pre-mRNA into mRNA occurs only in _______ of eukaryotic cells. It occurs on the ________.
|
the nucleus; spliceosome
|
|
|
A spliceosome is a complex of _____ and ______ that removes ______ from pre-mRNA in the nucleus.
|
snRNA; protein subunits; introns
|
|
|
Name five things that can cause denaturation.
|
1. Heat
2. changes in pH 3. changes in salt content 4. detergents 5. oxidants/reductants |
|
|
PRPsc is the abnormal prion protein. How/why does it replicate?
|
It can be genetic or ingested. The abnormally folded protein can get next to a normal protein and induce the formation of a homodimer. This process continues until an amyloid plaque of abnormal proteins develops.
|
|
|
Which residues typically undergo post-translational glycosylation? Is it permanent?
|
This covalent addition is relatively permanent. Ser/Thr are likely to undergo O-linked glycosylation, which Asn will undergo N-linked glycosylation.
|
|
|
What kind of permanent post-translational modification did we learn about? What examples were given?
|
Partial proteolysis. Zymogens. Insulin.
|
|
|
What effect do oligosaccharides have when added to proteins?
|
They are hydrophilic and thus increase protein solubility.
|
|
|
Glycoproteins created in the cell are destined to be....
|
either secreted or exposed on the cell surface.
|
|
|
Where does N-glycosylation occur? What sequence must be present?
|
In the lumen of the RER. The sequence must be Asn-X-Ser/Thr where X is not Pro or Asp.
|
|
|
Where does O-linked glycosylation occur? What sequence must be present?
|
In the Golgi. No sequence is necessary. Ser and Thr undergo O-glycosylation, as does an already hydroxylated Lys in collagen.
|
|
|
Lipids added post-translationally often serve as anchors in _____. What residues did we see examples of?
|
the cell membrane. C-terminal or internal Cys. N-terminal Gly.
|
|
|
What is a GPI anchor? Where would you be likely to find one? What purpose does it serve?
|
A GPI is glycosyl-phosphatidyl inositol, which is a lipid that is added to proteins as a post-translational modification near the C-terminus. The GPI/proteins are likely to be found in membrane raft areas. They allow proteins to be more mobile on the cell surface.
|
|
|
_______ occurs on the three amino acids containing a hydroxyl group (which are...). This is a reversible process.
|
Phosphorylation. Serine, threonine, and tyrosine.
|
|
|
Post-translational phosphorylation is catalyzed by_____. Dephosphorylation is catalyzed by ________.
|
kinases; protein phosphatases
|
|
|
EPO is....
|
erythropoietin, a performance enhancer than carries more red blood cells to muscles. It is a naturally occurring protein that is also available as a recombinant protein for the treatment of anemia.
|
|
|
How can we tell if the EPO is natural, or if athletes are doping?
|
Although the primary structure of EPO and rEPO is the same, the post-translational modifications are not. These modifications change the pI, so the two forms can be distinguished by isoelectric focusing.
|
|
|
In isoelectric focusing, proteins move across a ____ and a _____ until they reach their pI.
|
pH gradient (high to low); electric field (negative to positive)
|
|
|
Protein structures are obtained by using _____ or _____.
|
X-ray crystallography; NMR
|
|
|
Proteins work according to these two fundamental principles, which are often intimately linked.
|
Binding and conformational change.
|
|
|
The molecule bound to the protein is called the ____.
|
ligand
|
|
|
The affinity of a protein for a ligand is expressed as ______. It has units of _____.
|
The dissociation constant Kd; unit of concentration of ligand
|
|
|
Kd=
(two formulas) |
Kd = [Ligand(free)] * [binding sites(free)] / [protein-ligand]
Kd = kd/ka |
|
|
Kd is.....
A high Kd corresponds to _____. |
The concentration of ligand at which 50% of ligand will be bound.
High Kd = low affinity |
|
|
Two important things to remember about the Scatchard plot.
|
1. Bmax is the X-axis intercept.
2. slope = -Kd |
|
|
kd represents the ______ of a protein/ligand compound. A high kd corresponds to _____. (NB: kd /=Kd)
|
stability; a rapid rate of dissociation
|
|
|
What is the oxidation state of the heme iron? What residue does it form a bond with?
|
The iron is ferrous (reduced iron, Fe2+).
It bonds with histidine. |
|
|
The increased affinity of hemoglobin for oxygen after one O2 has bound is an example of _____.
|
allosteric (or cooperative) binding
|
|
|
What are the shapes of the myoglobin and hemoglobin oxygen affinity curves?
|
Myoglobin: hyperbolic
Hemoglobin: sigmoid |
|
|
What is the Bohr effect?
|
Describes how hemoglobin affinity for O2 decreases as:
1. pH decreases 2. CO2 increases 3. temperature increases 4. 2,3-BPG increases This means more will be released to myoglobin. |
|
|
In sickle cell, a normal _____ residue is replaced with a _____ residue.
|
glutamate; valine
|
|
|
The ELISA test is used to...
|
determine the presence of an antigen or antibody. (Used for HIV)
|
|
|
The Ompg porin is a channel that will be _____ at biological pH and will be _____ at lower pH.
|
open; closed
|
|
|
SNAREs are...
|
proteins involved in vesicle transport (exocytosis).
|
|
|
If A+B <----> C +D, then Keq=
|
Keq= [C][D]/[A][B]
|
|
|
DeltaG=
|
deltaG = -RTln(Keq)
|
|
|
If Keq > 1, the reaction is said to be...
|
favorable (exergonic)
|
|
|
If Keq < 1, the reaction is said to be...
|
unfavorable (endergonic)
|
|
|
The same catalyst molecule can catalyze many reactions. This is called ______.
|
turnover
|
|
|
This rxn is catalyzed by ______:
ATP+glucose ---> ADP + glucose-6-phosphate |
hexokinase
|
|
|
In gluconeogenesis, pyruvate + CO2 ----> _______. What must happen to make this rxn energetically favorable?
|
oxaloacetate; It must be couple to the hydrolysis of ATP. The enzyme pyruvate carboxylase makes this coupling possible.
|
|
|
This rxn is catalyzed by _____:
ATP + sulfate -----> adenosyl phosphosulfate +PPi |
pyrophosphatase
|
|
|
_____ as well as proteins can be enzymes. They are called ____. They are the part of the ribsome responsible for _______.
|
RNA; ribozymes; translation of RNA
|
|
|
Name the six major groups of enzymes.
|
Type 1: oxidoreductases
Type 2: transferases Type 3: hydrolases Type 4: lyases Type 5: isomerases Type 6: ligases |
|
|
Glyceraldehyde-3-phosphate dehydrogenase is know to be an enzyme that "moonlights," meaning....
|
it is know to have other functions than it's well-known role in glycolysis. In this case, it has many.
|
|
|
In the enzyme binding site, there are two kinds of residues: _____ and _____. There may also be _____ or metal ions.
|
binding; catalytic; cofactors
|
|
|
Regulatory sites where inhibitors or activators bind to an enzyme are called ______.
|
allosteric sites
|
|
|
What is a morpheein? What example were we given in class?
|
PGBS regulated by Mg2+. The morpheeins are the two different kinds of quaternary structure. PGBS w/o Mg2+ will exist as an inactive hexamer. W/ Mg2+, it will be an active octamer.
|
|
|
An enzyme without its cofactor is called a/an ______. An enzyme with its bound cofactor is called a/an ________.
|
apoenzyme; holoenzyme
|
|
|
In enzyme kinetics, k1 is...? k2? k3?
|
k1 is the rate of formation of ES from E+S
k2 is the rate of dissociation of ES to E+S k3 is the rate of formation of E+P from ES |
|
|
If there is much more substrate than enzyme, the the velocity of the formation of product is what?
|
v = k3 x [ES]
|
|
|
What is the formula for v for all substrate concentrations?
|
v = (Vmax[S])/(Km +[S])
|
|
|
What is the Lineweaver-Burk plot? What useful values does it provide?
|
It is the double reciprocal plot. (Reciprocal of Michaelis-Menton) The y-intercept = 1/Vmax. The x-intercept = 1/-Km.
|
|
|
In the conversion of threonine to isoleucine, _____ acts as an allosteric inhibitor early in the pathway. This is an example of _______.
|
isoleucine; feedback inhibition
|
|
|
Chymotrypsinogen is an inactive _____. It is activated by __________.
|
zymogen; proteolytic cleavage
|
|
|
Pyruvate carboxylase uses the cofactors ___ and ____, and has _____ as an allosteric activator. These molecules can modulate the ____ structure of pyruvate carboxylase.
|
biotin; Mg; acetylCoA; quaternary
|
|
|
How fast is the fastest enzyme?
|
Turnover is about 10^10/sec
|
|
|
Antibiotics:
Vancomycin and beta-lactam inhibit _____. |
formation of cell wall
|
|
|
Antibiotics:
Erythromycin and tetracyclin disrupt ____. |
ribosomes
|
|
|
Antibiotics:
Quinoline and sulfonamide inhibit _____. |
DNA synthesis/replication.
|
|
|
Penicillin type drugs are _______ antibiotics. They work by _________.
|
beta-lactam; inhibiting crosslinking of bacterial cell wall.
|
|
|
What is beta-lactamase? What can be used to combat beta-lactamase?
|
It breaks down beta-lactam antibiotics, giving bacteria immunity to penicillin. In this case, augmentin, a combination drug should be used. It contains both beta-lactam and an irreversible beta-lactamase inhibitor.
|
|
|
Define isoenzyme.
|
Isoenzymes are enzymes that differ in their primary sequence, but catalyze the same reaction. These enzymes usually display different kinetic parameters (e.g. Km values).
|
|
|
What is epigenetics?
|
The study of how factors other than DNA can change gene expression. In our case, I believe we are concerned with the packaging of DNA into chromatin.
|
|
|
About how many base pairs in the human genome?
|
~3.2 billion bp in a HAPLOID human genome.
|
|
|
What percentage of DNA codes for proteins? How many protein coding genes are there in a human?
|
<1.5%. There are around 25,000.
|
|
|
There are around 25,000 genes that code for protein in a human, but there are around 100,000 proteins. Explain.
|
The mRNA can be spliced in different ways to create different proteins. Also, the proteins can be post-translationally modified.
|
|
|
About how many base pairs will be different from human to human?
|
1/1000 bp
|
|
|
Name three disease susceptibility genes identified through the Human Genome Project.
|
Risk factors for:
1. Atrial fibrillation and stroke 2. Crohn's 3. myocardial infarction |
|
|
What are housekeeping genes?
|
They are expressed in most cells.
|
|
|
Name some genes that might be expressed in only a certain type of cell.
|
1. Hb in red blood cells
2. insulin in beta cells of pancreas |
|
|
How far would the human genome stretch if DNA was not coiled?
|
About 1 meter, meaning each chromosome would be about 4.3inches long.
|
|
|
DNA wrapped around a histone is called a _____.
|
nucleosome
|
|
|
Nucleosomes are coiled to form the _____ form. It is about ___ nm high.
|
solenoid; 30
|
|
|
The solenoid strand is bound to a protein ______.
|
scaffold
|
|
|
The width of naked DNA is about __ nm. The histones are __ nm. The solenoid form is __ nm. The solenoid on the scaffold is __ nm.
One chromatid is __ nm wide. Therefore, the chromosome is ___ nm wide. |
2,11,30,300,700,1400
|
|
|
What is condensin?
|
It is a multiprotein complex that plays a role in compacting DNA.
|
|
|
Are histone proteins basic or acidic? Why? How many protein subunits in a histone?
|
Histones are basic so they can interact with the acidic/negative backbone of the DNA. Histones are octamers (4 different subunits).
|
|
|
What does histone H1 do?
|
It promotes the coiling of nucleosomes into the solenoid form.
|
|
|
What kind of tails extend from a histone?
|
They are N-terminal tails. They may promote condensation.
|
|
|
Where will you find heterochromatn?
|
At the centromere and the telomeres. It is permanently condensed.
|
|
|
What is euchromatin?
|
During interphase, it is the portion of DNA that will have some uncoiled, actively-transcribed portions.
|
|
|
What do chromatin remodeling complexes do?
|
They loosen the coil to make some portions of DNA more open and therefore, more accessible to transcription factors.
|
|
|
Chromatin remodeling complex require ___ to function.
|
ATP
|
|
|
What kind of modifications can occur to Lys in chromatin?
|
N-terminal Lys can be methylated or acetylated. Looks like arginine can also be methylated
|
|
|
What kind of modifications can be made to Ser in chromatin?
|
Phosphorylation
|
|
|
What covalent chromatin modification creates a more open DNA structure, leading to more transcription?
|
acetylation
|
|
|
What proteins regulate acetylation of chromatin?
|
Histone DeACetylase (HDAC): removes acetyl group, coils histones more tightly.
Histone AcetylTransferase (HAT): adds acetyl groups, uncoils histones. |
|
|
What is a Barr body?
|
It is the inactive X chromosome in a female. Some cells express the maternal X, while some cells express the paternal X. This leads to mosaicism.
|
|
|
What causes the coiling/inactivation of the Barr body?
|
XIST RNA
|
|
|
Describe how Duchenne muscular dystrophy is expressed differently in males and females.
|
This is an X-linked recessive disorder. The male has only one X chromosome. All cells will have the mutant DNA and will express the disease.
The female is likely to be heterozygous, with only some cells expressing the mutant gene. |
|
|
What is a transcription unit?
|
The part of the gene that is transcribed into RNA (exons and introns included).
|
|
|
The gene that codes for this protein has 178 exons.
|
Titin
|
|
|
Where is a promoter? How big is it?
|
It is upstream (towards 5' end) from exon 1. It is 200-400 bp.
|
|
|
What types of RNA are the following polymerases associated with? Pol I, Pol II, Pol III
|
Pol I: rRNA
Pol II: mRNA Pol III: tRNA |
|
|
RNA is composed on the ______ strand (aka the template strand), meaning that but for the substitution of uracil, its sequence is the same as the ____ strand.
|
antisense; sense
|
|
|
What are these things:
TATA box TBP TFIID |
The TATA box is where transcription starts.
TATA box Binding Protein (TBP) binds to the TATA box. TBP is part of the larger Transcription factor II-D. |
|
|
Two things I'd like to remember that TFII's do.
|
1. Find the TATA box.
2. Attract Pol II |
|
|
What is an enhancer?
|
It promotes the transcription of a certain gene. It is the binding site of the activator, which can be quite far upstream from the site of transcription. Remember the DNA curves around so the activator can interact with the transcription factors. This contact sets Pol II flying.
|
|
|
What do regulatory transcription factors do?
|
They control the rate of transcription. They can be activators or repressors and are often tissue specific.
|
|
|
Describe the three important regions of most tx factors.
|
1. Activation domain: Where the factor interacts with other tx factors
2. DNA binding domain: Where is connects to the DNA. Usually an alpha-helix. Usually has basic amino acids. 3. Dimerization domain: I don't know what happens here, but it is hydrophobic and usually functions as a dimer. |
|
|
Describe the structure of TBP.
|
It is a single subunit. Unusually, it is a beta strand that lies in the minor groove. There are helical parts, but they interact with other tx factors.
|
|
|
What are the two tx factor DNA binding motifs we learned about?
|
the basic-zipper (bZip), and the basic helix-loop-helix (bHLH)
|
|
|
Describe the bHLH.
|
It looks like the bZip... but with loops.
Alpha helices contact DNA in the major groove. It is made of amphipathic helices that dimerize into a coiled coil by hydrophic interactions. |
|
|
Describe the bZip. One example of bZip motif.
|
Two straight alpha helices.
N terminus regulates binding to major groove. C terminus regulates dimerization. The Leucine Zipper is a bZip. So is AP1. |
|
|
Zinc finger as DNA binding motif.
|
Folds into a-helices and b-strands. The a-helix sits in the major groove.
|
|
|
How does the nuclear receptor family of transcription factors differ from the other dimer transcription factors?
|
They require a ligand.The ligands are small lipid-soluble hormones and vitamins.
|
|
|
What kind of tx factors will often moderate a gene's response to a hormone?
|
nuclear receptor transcription factors. The hormone can act as a ligand.
|
|
|
What are some hormones that activate nuclear receptor transcription factors? Where do they bind?
|
They bind to INTRACELLULAR receptors. The hormone-receptor complex enters the nucleus. Some examples: cortisol, aldosterone, testosterone, estradiol, etc.
|
|
|
What does MyoD do?
|
It controls skeletal muscle differentiation. (Myf5 and myogenin are also needed for myogenesis from somites.) bHLH motif. transcription factor.
|
|
|
What does Runx2 do?
|
It controls osteoblast differentiation. It is a Runt domain tx factor. It clamps the DNA with its C-terminal "tail" in the major groove, and its "wing" in the minor groove.
|
|
|
Cleidocranial dysplasia is due to mutations in _____.
|
Runx2
|
|
|
What is a basal complex?
|
Looks like a whole mess of tx factors that bind at the TATA box and around it. It interacts with many far upstream transcription regulators.
|
|
|
What is the structure of the mRNA cap?
|
It is 7-methyl-guanosine attached to the 5' end of the mRNA through a 5'-5' triphosphate bond.
|
|
|
How does the spliceosome know where the introns are?
|
Consensus sequences
|
|
|
Describe how the intron detaches.
|
A 2'OH somewhere in the intron attacks the 5' end of the intron, forming a little lasso attached to the 3' splice site. The upstream exon then attacks the still attached end of the intron and the intron is released.
|
|
|
Where do you find snRNPs? How do they relate to the consensus sequence of the intron?
|
They are in spliceosomes. They are complementary to consensus sequences.
|
|
|
What enzyme regulates the removal of introns.
|
It is actually the snRNA of the spliceosome that catalyzes this rxn. RNA acts as an enzyme.
|
|
|
Where is the polyA tail attached?
|
At the 3' end of mRNA after the consensus sequence AAUAAA
|
|
|
What enzyme links the amino acid to the 2' or 3' OH of the tRNA?
|
aminoacyl tRNA synthetase
|
|
|
AMP-aa +tRNA --> AMP + aa-tRNA
What reaction is this coupled to? |
ATP ---> AMP + PPi
PPi ---> 2Pi |
|
|
What are the three bonding sites of aminoacyl tRNA synthetases?
|
Amino acid, tRNA, and ATP
|
|
|
How many aminoacyl tRNA synthetases are there?
|
20. One for each amino acid.
|
|
|
How many subunits in a ribosome? What are the constituents of the subunits? Which subunit is the ribozyme?
|
There are two subunits, the large and the small. Each subunit has proteins and RNA molecules. The large subunit is the ribozyme, catalyzing peptide bond formation.
|
|
|
What type of proteins carry tRNA to the ribosome?
|
G proteins
|
|
|
The hydrolysis of _____ provides some of the energy for protein synthesis.
|
GTP
|
|
|
What are the three tRNA binding sites on a ribosome?
|
A. aminoacyl tRNA
P. peptidyl tRNA E. exit |
|
|
GTP binds to G proteins (tRNA carriers) to activate them. Describe this. There is one thing you should know.
|
The inactive G protein is coupled with GDP. Then, GDP is COMPLETELY REMOVED, and GTP is added. However, to deactivate the protein, GTP is simply dephosphorylated.
|
|
|
At which binding site do aminoacyl tRNAs enter the ribosome?
|
They enter at the A site, except the initiator tRNA, which codes for Met, which will enter at the P site.
|
|
|
What do G proteins do (relative to RNA translation)?
|
They bring tRNA to the ribosome.
|
|
|
What does a release factor do?
|
It enters the ribosome when it comes to a stop codon.
|
|
|
eIF, eEf, and termination factors. What do these do?
|
Eukaryotic initiation factors are involved in translation initiation. Eukaryotic elongation factor are involved in growing the polypeptide chain. Termination factors are involved in the release of the protein from tRNA.
|
|
|
What are two things that might cause a bit of mRNA to be translated less efficiently?
|
A suboptimal AUG consensus sequence.
Secondary structure in the 5' untranslated region. |
|
|
Eukaryotic mRNA forms circular structures due to the interactions between _____ and _____.
|
the 5' cap; the poly A tail
|
|
|
What is a benefit of the mRNA forming a circular structure?
|
It promotes reinitiation, meaning that after the protein is translated, it's 5' end will be positioned to bind the ribosome again.
|
|
|
How many high energy molecules are used during protein synthesis?
|
At least three for each amino acid (1 ATP, 2 GTP)
|
|
|
Name two viruses that modify eukaryotic translational machinery.
|
Diptheria and polio.
|
|
|
Streptomycin, tetracycline, erythromycin, and chloramphenicol all inhibit bacterial translational machinery by binding where?
|
At the interface of the two ribosomal subunits.
|
|
|
In which direction are proteins synthesized? Relate this to the reason some ribosomes are attached to RER.
|
N-terminus to C-terminus. The N-terminus has a sequence on it that will tell the ribosome to attach to the ER.
|
|
|
Describe the signal sequence on the N-terminus of proteins destined for the secretory pathway.
|
There will be one or two basic residues (R or K) followed by 15-25 mostly hydrophobic residues). It will be removed by signal peptidase.
|
|
|
What is ubiquitin?
|
It tags proteins for destruction. It's a protein that attaches to lysine. The tagged protein gets degraded by proteasomes and the ubiquitin is recycled.
|
|
|
Where does ubiquitinylation/proteasome degradation take place?
|
In the cytoplasm.
|
|
|
What two essential fatty acids are used as a starting point for the the others?
|
alpha-linolenic acid and linoleic acid
|
|
|
Describe the omega naming convention for fatty acids.
|
Omega is the last carbon on the fatty tail. If a FA is "omega-3" that means that there is a double bond at the third carbon from omega.
|
|
|
What are triacylglycerides?
|
TAGs are the storage form of fatty acids. They have been esterified by addition of glycerol. There is no hydrophilic portion.
|
|
|
List the components of a phospholipid.
|
Glycerol backbone attached to two FA tails and one phosphate group. A hydrophilic group is attached to the phosphate.
|
|
|
How do sphingolipids differ from phospholipids?
|
Instead of a glyerol backbone, they have a sphingosine backbone. Also, the FA on the second bond is linked through a CO-NH bond.
|
|
|
What is the head group of sphingomyelin? Where might you find it?
|
Phosphocholine. Essential component of myelin sheaths. Associated with membrane rafts.
|
|
|
What are anchored membrane proteins?
|
They are proteins with a lipid tail that connects them to the membrane. They are more mobile than transmembrane proteins.
|
|
|
What is a GPI anchor? How is it attached? Where does the attaching take place?
|
It is a lipid tail that anchors a protein in a cell membrane. It is attached in the lumen of the ER by cleaving a couple of residues on the C-terminal and attaching there.
|
|
|
Where do GPI anchored proteins often accumulate?
|
Cholesterol rich microdomains (membrane rafts)
|
|
|
Name some things you will probably see in membrane rafts.
|
Lipids with saturated tails, GPI-anchored proteins, cholesterol, sphingolipids
|
|
|
Name some functions of a lipid raft.
|
Signaling platforms, exocytosis, targets for pathogens
|
|
|
What are flippases, floppases and scrambleases? Which is ATP-independent?
|
They facilitate transverse diffusion across the phospholipid membrane. Scrambleases are dependent on calcium, not ATP.
|
|
|
Where would you find a phosphoinositide?
|
Anchored on the INSIDE of a cell in the cell membrane.
|
|
|
What does a phosphoinositide do?
|
When it is cleaved from the cell membrane in the form of inositol 3-phosphate (IP3), it activates the release of Ca2+.
|
|
|
Which human organelles have a double membrane?
|
mitochondria and nucleus
|
|
|
What do dynamin and ESCRT have in common?
|
Both enzymes for membrane fission.
|
|
|
When just the outer leaflets of two membranes have fused, this is referred to as ______.
|
hemifusion
|
|
|
What are SNAREs?
|
They are intracellular enzymes that facillitate membrane fusion. There is a SNARE in both membranes to be fused.
|
|
|
What are liposomes?
|
Artificial vesicles made from phospholipids and cholesterol. Used to deliver drugs.
|
|
|
Why are liposomes well-suited for drug delivery?
|
They are biocompatible, non-immunogenic, non-toxic, and have a long half life when injected.
|
|
|
What are the 4 kinds of cell signaling?
|
1. endocrine
2. paracrine (incl. autoparacrine) 3. neuronal 4. contact-dependent |
|
|
What effect does acetylcholine have on heart muscle? Salivary glands? Smooth muscle?
|
1. decreased contraction
2. secretion 3. contraction (The heart muscle and salivary glands have the same receptor.) |
|
|
Extracellular signals can change cell function slowly or rapidly. Which kinds of signals produce a rapid change?
|
If a signal alters protein function, it will alter cell behavior rapidly. If a signal induces the transcription of DNA to make new proteins, change will be slower.
|
|
|
Cortisol, etradiol, testosterone, thyroxine. What do these have in common?
|
They are cell signalers that act on intracellular receptors.
|
|
|
What are GPCRs?
|
G-protein coupled receptors in the cell membrane. They have 7 transmembrane domains.
|
|
|
What happens when a GPCR binds to its extracellular ligand?
|
It will activate the G-protein, which will then activate a number of membrane proteins, including adenylyl cyclase and phospholipase C.
|
|
|
Which enzyme sythesizes cAMP? What degrades cAMP? What is cyclic AMP?
|
Adenylyl cyclase. Phosphodiesterase. cAMP is a second messenger.
|
|
|
What are some hormones that use cAMP as their second messenger?
|
Adrenaline, ACTH, glucagon
|
|
|
Describe how adrenaline stimulates glycogen breakdown.
|
Adrenaline > GPCR > G-protein > adenylyl cyclase > cAMP > PKA > phosphroylase kinase > glycogen phosphorylase > glycogen breakdown
|
|
|
What are enzyme coupled receptors?
|
When an extracellular ligand binds to them, their separate parts are brought together and activated.
|
|
|
Which enzyme couple receptor did we learn about?
|
Receptor tyrosine kinase (RTK). When the ligand binds, multiple phosphoryl groups attach to the tyrosine. Signaling proteins then bind to the phosphotyrosines.
|
|
|
When receptor tyrosine kinase is activated, what do the signals that is sends do? (There are many answers, but which one did we learn about?)
|
They can activate the G protein, Ras. They are the receptor for insulin.
|
|
|
What cascade will the G protein Ras activate?
|
It will activate a MAP kinase cascade that will both change protein activity and gene expression.
|
|
|
Which type of receptors is responsible for transmission of signals across synapses?
|
Ion-channel coupled receptors, like the one for acetylcholine.
|
|
|
What structure can hold inactive signaling proteins in closer proximity to the receptor protein?
|
A molecular scaffold.
|
|
|
What does an integrator protein do?
|
It incorporates signals from multiple pathways before passing the signal onward.
|
|
|
All mammalian steroid hormones are formed from cholesterol
through a common intermediate ________. |
pregnenolone
|
|
|
Tri-iodothryronine (T3), thyroxine (T4), epinenphrine, and norepinephrine are all derivatives of _______.
|
tyrosine
|
|
|
How is insulin activated from its prohormone state?
|
A middle swath is cleaved and the two ends form disulfide bonds with one another.
|
|
|
What kind of receptor is the insulin receptor?
|
It is a receptor tyrosine kinase (RTK).
|
|
|
What kind of receptor is the receptor for glucagon?
|
A GPCR
|
|
|
What are integrins?
|
They are receptors that mediate a cell's attachment to other cells or to the ECM.
|

