![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
131 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Describe the chromosomal organization of the duplex human genome.
|
•23 pairs of homologous chromosomes- 46 total, diploid (2n)
•homozygous vs heterozygous (know the difference) •how to visualize chromosomes? -giemsa staining vs spectral karyotyping (what is the difference?) -can be used to reveal chromosomal translocations •Cytogenetics- using chromosome number and structure to diagnose disease •Structure of generic mitotic chromosome |
|
|
|
Diagram how DNA is packed into chromosomes.
|
•Tightly packed around nucleosomes to form chromatin
onucleosomes are composed of DNA wrapped around histones -linker + wrapped DNA ~200 bps -histones are basic -modifications of specific histone residues regulates transcription/replication •Euchromatin- loosely packed, and transcriptionally active •Heterochromatin- densely packed, usually transcriptionally inactive •10,000x compaction |
|
|
|
Note key factors and DNA replication structures
|
•Know how replication errors are corrected
-3’ exonuclease removes incorrect bases •Understand rationale underlying ciprofloxacin: -To relieve supercoiling when DNA is unwound: -Eukaryotes use topoisomerase -Prokaryotes (bacteria) use gyrase •Ciprofloxacin inhibits gyrase, which has no homology to topoisomerase |
|
|
|
Define and distinguish SNPs and STR polymorphisms.
|
•Polymorphisms- mutations that distinguish individuals
SNP = single nucleotide polymorphism. Single nucleotide polymorphisms (SNPs) are simply sequence variations between individuals at a particular point in the genome. STR: short tandem repeat (STR) in DNA occurs when a pattern of two or more nucleotides are repeated and the repeated sequences are directly adjacent to each other. The pattern can range in length from 2 to 5 base pairs (bp). prevalent analysis method for determining genetic profiles in forensic cases. •>50% of human genome is comprised of repetitive sequence |
|
|
|
Describe some key points of translation - at the mRNA level (part I)
|
•Transcription:
-Synthesized RNA in 5’ to 3’ direction -Can occur on either Watson or Crick strand -Promoter regulates directionality of transcription -Regulatory elements determine levels of RNA expression through binding of activators or repressors -3 kinds of RNA Pol -I- rRNA - II- mRNA, snRNAs, miRNAs -III- tRNA, snoRNAs |
|
|
|
Describe some key points of translation - at the protein level (part 2)
|
oDon’t memorize genetic code! Understand which codon positions, when mutated, change the identity of the amino acid. Hint—1st and 2nd.
-Know stop codons—UGA, UAA, UAG -tRNAs directly translate nucleic acid information to amino acid information |
|
|
|
Diagram tRNA, identifying the anticodon and site of aminoacylation.
|
•understand interaction of anticodon with codon, be able to write anticodon in 5’-3’ orientation
•tRNA bases are heavily modified (slide 26) -don’t memorize structures of modifications, just know that they are modified -underlies the cause of MELAS (Myopathy, Encephalopathy, Lactic Acidosis, Stroke-like episodes) -mitochondrial cause—maternally inherited |
|
|
|
Give an example, the mode of action and the resistance mechanism of: Protein Synthesis antibiotics
|
Example:Macrolides
Mode of Action: Block peptidyltransferase reaction Resistance Mechanism: rRNA methylation |
Resistance Mechanism:
rRNA methylation |
|
|
Give an example, the mode of action and the resistance mechanism of:
Protein Synthesis antibiotics |
Example:Tetracycline
Mode of Action: Block peptidyltransferase reaction Resistance Mechanism: drug efflux |
Resistance Mechanism:
drug efflux |
|
|
Give an example, the mode of action and the resistance mechanism of:
Protein Synthesis |
Example:Aminoglycosides (Neomycin)
Mode of Action: Block peptidyltransferase reaction Resistance Mechanism: enzymatically modify drug |
Resistance Mechanism: enzymatically modify drug
|
|
|
Give an example, the mode of action and the resistance mechanism of:
DNA replication/repair antibiotics |
Example: Fluoroquinolones(ciprofloxacin)
Mode of Action:DNA gyrase (bacterial topo II) gyrase mutations (ciprofloxacin) chain break and rejoining Resistance Mechanism: gyrase mutations |
Resistance Mechanism: gyrase mutations
|
|
|
Give an example, the mode of action and the resistance mechanism of:
DNA replication/repair antibiotics |
Example:Novobiocin
Mode of Action: DNA gyrase binding to ATP Resistance Mechanism:gyrase mutations |
Resistance Mechanism:gyrase mutations
|
|
|
Give an example, the mode of action and the resistance mechanism of:
Cell Wall antibiotics |
Example: beta-lactams (Ampicillin)
Mode of Action:Block crosslinking enzymes Resistance Mechanism: β-lactamases destroy drug |
Resistance Mechanism: β-lactamases destroy drug
|
|
|
Give an example, the mode of action and the resistance mechanism of:
Cell Wall antibiotics |
Example:Vancomycin
Mode of Action:Sequester substrate for crosslinking Resistance Mechanism: make different substrate |
Resistance Mechanism: make different substrate
|
|
|
What to know about regulation of transcription of human genes (part one)
|
•Chromatin structure
-Determines accessibility of DNA to RNA Pol -Histones are modified at specific residues (HATs, HMTs) -Acetylation tends to be activating -Deacetylation and Methylation tend to be repressive -Histones are specifically positioned along length of DNA (chromatin remodelers)—wrapping DNA around histones is inherently repressive because of diminished accesibility |
|
|
|
What to know about regulation of transcription of human genes (part two)
|
•Promoter strength (how well RNA pol and general txn factors bind)
•Cis-regulatory elements (which are bound by activating or repressive trans-factors) -Trans-factors have specific DNA-binding motifs -HLH and leucine zippers •CpG methylations -typically silences genes -only some CpG dinucleotides are methylated -CpG methylation pattern is maintained heritably -Deamination of 5-met-C leads to T -Pattern is heritable |
|
|
|
List the symptoms of Rubinstein-Taybi syndrome, and identify the molecular deficiency that causes this disease.
|
•Mutation in CREBBP, a HAT, underlies Rubinstein-Taybi syndrome
-Facies -Broad thumbs and first-toes -Short, with small heads -Cryptochordism -Developmental defects (mental disability, poor coordination) |
|
|
|
What are the the symptoms caused by deficiencies in the steroid hormone testosterone and its receptor
|
Defects in secondary sexual characterisics, won’t respond to steroid replacement therapy if Receptor is mutated.
|
|
|
|
Identify the mechanism by which high dose aspirin therapy is effective in treating rheumatoid arthritis, and the limitations of this treatment.
|
•Irreversibly inhibits COX-1, leading to decreased inflammation
•GI bleeding from inhibition of thromboxane (necessary for clotting) |
|
|
|
Distinguish amino acids commonly found on the exterior and interior of folded proteins.
|
•Interior- hydrophobic
•Exterior- hydrophilic |
|
|
|
List the symptoms of Alzheimer's disease, and diagram characteristic damage to the brain.
|
•Symptoms: memory loss, dementia, personality change
•Damages: amyloid plaques and neurofibrillary tangles containing Tau. oUnderstand how amyloid plaques arise |
|
|
|
Define a prion, and outline how a prion can be responsible for familial, iatrogenic or infectious disease.
|
Creutzfeldt-Jakob disease (CJD)- familial, sporadic or iatrogenic
•rapidly progressive dementia, mean duration 6 mo. •communicable via corneal transplant, surgical instruments •strategy: sterilize instruments with alkali, not heat Variant BSE, also known as variant CJD (vCJD) — •from contaminated beef •all affected individuals carry PRNP polymorphism Kuru — infection through cannibalism Huntington disease phenocopy •due to repeat expansion within PrP coding region Gerstmann-Straussler syndrome (GSS)— familial Fatal familial insomnia (FFI), and sporadic familial insomnia |
|
|
|
Why it is misleading to describe Crick's "Central Dogma" as "DNA makes RNA makes protein"?
|
Only mRNA serves to make protein after being transcribed from DNA. RNA has many other roles in the cell, though.
|
|
|
|
How can alternative polyadenylation generate different proteins from one gene, and regulate translation of mRNAs encoding the same protein?
|
•Alternative polyadenylation can lead to different exons being included in the final mRNA (different proteins).
•Alternative polyadenylation can regulate 3’UTR sequence, resulting in differential binding by miRNAs and siRNAs (translation regulation) |
|
|
|
Describe how NMD (nonsense mediated decay) distinguishes a nonsense codon from a normal termination codon.
|
•“Pioneering round of translation”—to make sure mRNA contains useful information
•downstream exon-junction-complex (EJC) detects upstream, premature stop-codons |
|
|
|
Describe how the iron binding enzyme aconitase regulates translation of the ferritin and transferrin receptor mRNAs in opposite directions.
|
[Fe] = High --> Up[Ferritin], down[Transferrin]
[Fe]=Low --> Down[Ferritin], Up [transferrin] Ferritin sequesters excess Fe, while Transferrin imports extracellular Fe. |
|
|
|
List the 3 most basic principles of RNA folding.
|
1.Form all local helices
2.Stack adjacent helices on top of each other 3.Allow loops in local units to interact with each other |
|
|
|
What is a ribonucleoprotein particle?
|
Complexes composed of RNA + Protein.
|
|
|
|
Give at least 1 and perhaps 3 reasons why the ribosome is a ribozyme.
|
1. Active site composed of RNA
2. Antibiotics (Competitive inhibitors) bind the active site. 3. Evolutionary conservation of 23S rRNA peptidyl transferase center |
|
|
|
How can the structure of the ribosome guide design of new antibiotics?
|
Locate “pockets” for small molecules to fit and inhibit catalysis.
|
|
|
|
Describe the roles of small ribozymes in replication of RNA viroids
|
Hepatitis delta—genome is replicated into a linear, contiguous segment containing multiple genomes. Separating each genome is a self-cleaving ribozyme, resulting in multiple monomeric units
|
|
|
|
How is hepatitis d virus (HDV) a parasite of hepatitis B virus (HBV)?
|
HDV needs HBV proteins to package genome into virions—coinfection is required.
|
|
|
|
How are RNA hydrolysis, ligation, isomerization, splicing, and polymerization are all chemically similar?
|
Nucleophilic attack by hydroxyl (OH-)
If H2O is source of OH-, then hydrolysis occurs If RNA molecule is source of OH-, isomerization occurs. During ligation, a dehydration occurs (loss of H2O) |
|
|
|
Why might we expect a mutation in the basic mRNA splicing apparatus to be a embryonic lethal?
|
Almost all human genes are spliced. Mutating a basic splicing protein would result in universal splicing defects.
|
|
|
|
Mutations in proteins responsible for 3' splice site function often cause myelodysplasias. Describe how mutations in the basic splicing apparatus might cause developmental- or cell type-specific disease.
|
Almost all human genes are spliced. Mutating a basic splicing protein would result in universal splicing defects.
|
|
|
|
Give 2 or 3 reasons why the spliceosome is a ribozyme
|
1. Group II introns (which are in protists and bacteria), composed only of RNA, are almost chemically identical to splicing of human pre-mRNAs. (evolutionarily conserved)
2. The RNA components (not the protein components) of snRNPs are what interact with pre-mRNA |
|
|
|
List 4 or more reasons why antisense RNA therapy, although so simple in concept, is so difficult in practice.
|
• RNA molecules are large
• Off-target effects • RNA is inherently unstable. • RNA is repelled by lipid bilayer of cell membranes. |
|
|
|
Give at least 2 reasons why induction of exon skipping by antisense RNA or PNA has limited therapeutic potential for missense and nonsense mutations, as well as small insertions and deletions.
|
Very difficult to distribute RNA to entire body; can only administer locally as of right now.
Every case of genetic disease occurs in a different genetic background; such differences may affect stability, duration, uptake etc. of RNA (or PNA) molecules. |
|
|
|
Describe the different kinds of mutation that contribute to genetic disease including transitions, transversions, deletion, insertion, repeat expansion, repeat contraction, copy number variation and translocation
|
• Transition- purine to purine or pyrimidine to pyrimidine mutation
• Cytosine deamination- a cause of C to T transition mutation • Transversion- purine to pyrimidine, pyrimidine to purine • Deletion/insertion- loss or gain of a base-pair • Repeat expansion/contraction- gain or loss of short repeat sequences during replication • Copy Number variation- differences in the number of copies of a particular genetic locus • Translocation- the movement of a segment of DNA from one locus to another • Error rate of DNA replication = 10e-9, 1 error for every billion nucleotides added. |
|
|
|
Distinguish the kinds of mutations associated with replication error, spontaneous DNA damage, UV damage, ionizing radiation, and non-allelic homologous recombination
|
Replication error- transitions and transversion
UV damage- pyrimidine dimers Ionizing radiation- dsDNA breaks Non-allelic homologous recombination leads to a gain of DNA on one homologous chromosome, and loss of DNA on the other homologous chromosome. |
|
|
|
Describe how formation of a structure by a trinucleotide repeat may lead to repeat expansion or contraction; and to name two diseases caused by trinucleotide repeat expansion.
|
Hairpins can form when the newly synthesized DNA (red, left) re-hybridizes, or when the parental strand of DNA (red, right) re-hybridizes.
Huntingtons, Fragile X, |
|
|
|
What types of mutations occur in collagen genes and
what disorders do they produce? |
COL1A1
Nonsense mutations—OI type I Substitutions for glycine in triple helix—OI Splice site mutations—OI, EDS type VII Non-glycine missense mutations—Caffey disease EDS type I with aneurysms, aneurysms COL1A2 Nonsense mutations—EDS with cardiac valvular abnormalities Substitutions for glycine in triple helix—OI Splice site mutations—OI, EDS type VII |
|
|
|
What disorders of modifications produce clinical
alterations but are not lethal? |
N-propeptide cleavage (substrate and enzyme—
EDS type VII or dermatosparaxis) C-propeptide cleavage (substrate—increased bone density and ectopic calcification) Lysyl hydroxylation in helix (EDS type VI) Telopeptide lysyl hydroxylation (Bruck syndrome) Prolyl 3-hydroxylation (moderate to severe OI) |
|
|
|
What post-translational modifications are essential
for survival? |
Prolyl 4-hydroxylation (unless you are C. elegans)
|
|
|
|
What is important for molecular assembly?
|
Completion of synthesis
Hydroxylation of Y-position prolyl residues (Gly-X-Y) Folding of C-terminal propeptide Disulfide bond formation to stabilize conformation Chain recognition Triple helix nucleation Triple helix propagation Interaction with important chaperones or “grooming” molecules |
|
|
|
What are the characteristics of collagens?
|
-Homo- or heterotrimers
-Triple helix with glycine in every 3rd position (Gly-X-Y) 4-hydroxyproline, hydroxylysine -Synthesized as precursor -Secreted and processed, usually in matrix -Forms large scale aggregates (fibrils and other) |
|
|
|
Where are the proteins made?
|
Nuclear genes
Transcribed, spliced in the nucleus Translated on RER bound ribosomes Pre-proα chains inserted into the RER Co-translational modification of prolyl and lysyl residues Association of chains at carboxyl-terminal sites Triple helical propagation from carboxyl- to aminoterminal end Folded with associated proteins Secreted through the Golgi to the extracellular space |
|
|
|
What are the characteristics of collagens?
|
Homo- or heterotrimers
Triple helix with glycine in every 3rd position (Gly-X-Y) 4-hydroxyproline, hydroxylysine Synthesized as precursor Secreted and processed, usually in matrix Forms large scale aggregates (fibrils and other) |
|
|
|
What is important for molecular assembly?
|
Assembly involves many “helper” proteins
Protein disulfide isomerase (PDI) Prolyl 4-hydroxylase Prolyl cis-trans isomerase |
|
|
|
What are TERTs
|
TERT = protein component, catalyzes addition of dNTPs
|
|
|
|
What are TERCs
|
TERC = RNA component, acts as template for addition of dNTPs
|
|
|
|
Explain the connection between telomere length, cell senescence and physiological aging.
|
When telomeres are too short, the cell stops dividing (senescence.)
Telomere length is longer in children, shorter in adults. |
|
|
|
Distinguish alopecia, dyskeratosis and anemia, and explain why each can be caused by diminished telomerase activity or telomere function.
|
Alopecia- baldness
Dyskeratosis- autosomal cause is due to mutation in TERC. There is also an x-linked version, which is due to mutatin in dyskerin. |
|
|
|
Distinguish cancer cells from normal cells.
|
Benign- Growth is contained within a single mass of dividing cells
Malignant- cells have ‘broken loose’ to for secondary growths distant from site of origin. |
|
|
|
Distinguish oncogenes and tumor suppressor genes.
|
Oncogenes- tend to promote growth or cell division
Transcriptional activators Growth factors Cell cycle inducers Signaling factors Tumor suppressors- inhibit growth/cell division, induce apoptosis, prevent migration, maintain genomic integrity |
|
|
|
Outline mechanisms by which oncogenes and tumor suppressor genes contribute to cancer.
|
Oncogenes- tend to promote growth or cell division
Transcriptional activators Growth factors Cell cycle inducers Signaling factors Tumor suppressors- inhibit growth/cell division, induce apoptosis, prevent migration, maintain genomic integrity |
|
|
|
Identify the cause of the familial cancer, HNPCC.
|
HNPCC- mismatch repair deficient MSH2, MLH1, PMS2 To identify the cause of the genetic disease, Xeroderma pigmentosum.
|
|
|
|
Identify a common cause of gastric cancer, and explain how this cancer is treated.
|
Colorectal cancer, treated with resection or chemotherapy.
|
|
|
|
Explain the molecular mechanism by which imatinib (Gleevec) works.
|
Competitively inhibits Tyrosone Kinases (like ABL)
|
|
|
|
Describe the distinct roles of tamoxifen and aromatase inhibitors in treatment of ER+ breast cancers.
|
Rationale: tumor grows because of estrogen induced growth signals
Tamoxifen is estrogen analog, binds to estrogen receptor but does not allow coactivator binding. Aromatase inhibits conversion of testosterone to estradiol (the main estrogen in the body) |
|
|
|
List reasons why S phase and M phase cell cycle checkpoints are important in preventing cancer.
|
S phase checkpoint- want DNA to replicate error free
M phase checkpoint- want proper chromosomal alignment before cytokinesis |
|
|
|
Discuss the changes in organization of the chromosomes and spindle from mitotic prophase to cytokinesis.
|
As mitosis progresses, chromosomes condense, becomes bi-oriented, and attach to spindle poles through kinetochores. Upon cytokinesis, kinetochores help catalyze the separation of sister chromatids.
|
|
|
|
Discuss the positive and negative effect of cyclins, Cdk inhibitors (CdkIs), and Cdk-activating kinases (CAKs) on the activity of cyclin-dependent kinase (Cdk).
|
Cdks rise throughout S-phase and into mitosis, before falling as mitosis completes.
Effect on CdKs: Cyclins- positive CdkIs- negative CAKs- positive |
|
|
|
Discuss how E2 and E3 ligases ensure that ubiquitin monomers are added to specific target proteins but not to others
|
E2s and E3s (of which there are many copies in the genome) catalyze to ubiquitination (and subsequent degradation) of many protein substrates. Specificity for the substrate is determined by which E2 and E3 is used.
|
|
|
|
What is the purpose of having 100s of E2's and 1000s of E3's that can assemble into SCF complexes?
|
Protein substrate specificity- indiscriminate destruction of proteins is not desired.
|
|
|
|
List the main differences between apoptosis, autophagy, and necrosis.
|
Apoptosis- Deliberate, programmed cell death.
Features of apoptosis- blebbing, shrinkage, nuclear fragmentation, chromatin condensation Autophagy- highly regulated process where cell digests itself using lysosomes. Necrosis- Uncontrolled cell death List the main reasons why the lysosome, the proteasome, the GroEL/GroES "foldasome", and the mitochondrion are separate architectural compartments within the cytoplasm instead of free floating enzymes. |
|
|
|
Speculate about why mitochondria play such a central role in apoptosis.
|
Mitochondria are the energy centers of the cell, and can be thought of as reflective of the overall health of the cell. When mitochondria become dysfunctional, it’s time to apoptose. (pure speculation)
|
|
|
|
Discuss renewal and pluripotency of stem cell.
|
Pluripotency - ability to differentiate into many cell types
Renewal - proliferate indefinitely, maintain undifferentiated state |
|
|
|
To list five possible applications for stem cell therapy in treatment of human disease.
|
1,2,3. Hematopoietic stem-cell transplant (cancers, disease due to chemotherapy/irradiation, hematopoietic disease).
4.Parkinson’s 5.Type I Diabetes |
|
|
|
Discuss how an autologous iPS cell has been used to treat sickle cell disease in the mouse.
|
1. Make iPS cells from mouse fibroblasts
2. Correct sickle-cell mutation 3. Differentiate corrected iPS cells into hematopoietic lineage 4. Transplant into mice |
|
|
|
Discuss how virus structure affects entry and exit from the cell
|
Receptors on surface of virion dictate mechanism of entry
Presence/absence of envelope dictates budding/lysis respectively |
|
|
|
Describe how docking and entry control cell and tissue specificity of infection
|
Viruses bind to specific receptors, enter the cell by various pathways,
establish latent or lytic infection, multiply slowly or rapidly, and may escape by budding or lysis |
|
|
|
Describe some ways in which HIV-1 evades our immune system
|
Glycosylation sites on gp120 and gp41 cover host/virus interface, preventing antibody binding.
CD4 binding site of gp120 is in deep, molecular crevice High mutation rate. |
|
|
|
Describe the main diagnostic methods for viral disease
|
PCR
|
|
|
|
Describe how small molecules or peptides can interfere with viral life cycles
|
Oseltamivir (drug) and tetherin (human defense peptide) prevent release of Flu and HIV release from cell, respecively
|
|
|
|
Explain why useful antiviral therapies need not stop a virus dead in its tracks but only slow it down.
|
Allow time for host immune system to “catch up.”
|
|
|
|
Name features of virus life cycle that are key to virus classification.
|
Classified based on genome composition/structure on resulting replicative mechanism
|
|
|
|
Distinguish lytic and latent infection.
|
Lytic- Virus replicates and exits, resulting in lysis of host cell
Latent- Virus is present, but dormant May integrate into genome (virally encoded integrase/host recombination factors), or exist as extrachromosomal “episome” |
|
|
|
Explain how poliovirus infection shuts off host cell translation
|
eIF4 needed for host translation to recognize host mRNAs
polio degrades eIF4, allowing host ribosomes to work exclusively for polio, whose mRNA has no cap, but does have IRES |
|
|
|
Discuss the function of the HIV protease in the HIV life cycle.
|
Protease needed to cleave poly-protein at consensus sequence
|
|
|
|
Identify the steps in the HIV life cycle that are current targets for therapies.
|
1. Can use chain terminators to inhibit reverse transcription (Nucleoside analogs, nucleotide analogs)
2. Inhibit reverse transcriptase- Non-nucleoside RT inhibitors (NNRIs) 3. Inhibit protease |
|
|
|
Distinguish the functions of Ig V and C regions.
|
(V)ariable region makes contact with antigen
(C)onstant region determines class of antibody and underlies antigen removal mechanism. |
|
|
|
Describe how translocation activates the c-MYC gene in Burkitt lymphoma.
|
T8;14. During class-switch, IgH will fuse to c-myc, resulting in activation of c-myc expression. (All b-cells highly express IgH)
|
|
|
|
What is the difference between somatic and germline gene therapy.
|
Somatic gene therapy = not heritable
Germline gene therapy = heritable |
|
|
|
Describe how insertional mutagenesis can occur in the course of retroviral gene therapy.
|
If introduction of transgene results in random integration into genome.
Insertional mutagenesis evident as clonal populations of T cells, which diminish in response to treatment |
|
|
|
Describe how colposcopy can be used to diagnose cervical cancer. What is a Papanicolaou smear?
|
Culposcopy with acetic acid treatment- lesions present with ‘acetowhite’ epithelium with punctate pattern
Pap smear- A method used to screen for the cellular changes that accompany HPV infection and used as a diagnostic for HPV-induced disease. |
|
|
|
Describe the episomal and integrated forms of HPV.
|
Episome is just an extra-chromosomal piece of DNA that can replicate independently from host-DNA (non-integrated)
|
|
|
|
Distinguish a plantar wart and genital wart caused by HPV.
|
Plantar wart- wart on sole of foot or toe. Cause by HPV type 1.
|
|
|
|
List the genetic and pathophysiological features that distinguish low risk and high risk HPV types.
|
“High-risk” HPV types have active E6 and E7 genes, which are virally encoded oncogenes. E6 works by inhibiting p53 and preventing apoptosis. E7 promotes the degradation of Rb (through poly ubiquitination.)
|
|
|
|
List some strengths and weaknesses of therapies based on antisense RNA.
|
Strengths:
1. Could be highly specific for gene of interest 2. Potentially less side effects because no other genes affected (theoretically) Weaknesses: 1. Hard do distribute systemically. 2. How to get cells to take it up (RNA is very large) 3. Expensive. |
|
|
|
List the steps by which nascent RNAs can target siRNA-guided RISC-like complexes to silence chromosomal targets. How might this serve as the “genome’s immune system”?
|
Nascent RNAs will bind RISC-siRNA complexes, resulting in the silencing of chromatin overlapping the given locus. If this happens at an L1, it will prevent subsequent retrotransposition, acting as the genome’s immune system.
|
|
|
|
Discuss the role of the competing endogenous linc-MD1 ceRNA in the induction of muscle-specific genes.
|
Linc-MD1 sequesters miR-135 and miR-133 to prevent binding of the miRNAs with MEF2C and MAML1 mRNAs. As a result, translation of MEF2C and MAML1 is activated, resulting in the transcription of muscle genes.
|
|
|
|
Why might a mutation in a ceRNA like SERINC1 have the same effect as a PTEN mutation in progression to melanoma?
|
ceRNAs like SERINC1 quench miRNAs. If they are mutated, then this frees miRNAs to bind PTEN, resulting in decreased PTEN activity.
|
|
|
|
Why might introduction of a lincRNA into a somatic cell have the same effect as introduction of Oct4, Sox2 and Nanog mRNAs?
|
lincRNAs can act as scaffolds to bind complexes responsible for specific histone marks. By regulating where the lincRNA/protein complex particle binds, it can regulate (potentially) pluripotent-like transcriptional programs.
|
|
|
|
Define enzyme replacement therapy.
|
Injection of Recombinant Enzyme on a regular basis.
|
|
|
|
List some criteria which a panel of health care professionals may apply when deciding whether to recommend that all newborns should be screened for a specific disease.
|
1. A treatment for the disease should be available.
2. Early initiation of treatment leads to a better outcome. 3. The disease should not be too rare. 4. A reliable assay is available for newborn screening. |
|
|
|
Define a lysosomal storage disease, and provide two examples (names, symptoms).
|
Genetic (sometimes single mutated gene) diseases that result in dysfunctional lysosomes, leading to abnormal metabolism of lipids, glycogen, glycoproteins, etc.
Two examples: Pompe’s, signs and symptoms: Cardiomegaly, respiratory distress, hypotonia Fabry’s, signs and symptoms: Localized pain in extremities, renal failure, cardiomyopathy |
|
|
|
List the symptoms of Pompe disease, noting those that distinguish it from other hereditary diseases that we have studied this quarter.
|
Pompe’s is a form of muscular dystrophy. Enzyme replacement therapy can treat it very well, compared to other forms of muscular dystrophy.
|
|
|
|
Blood is a very complex mix. Explain how mass spec distinguishes metabolites of a specific substrate from the many other molecules in blood.
|
As long as the mass of the metabolite you are looking for is known, mass spec can detect it. Based on the combination of masses detected from fragments (generated by collision with Ar), your metabolite can be distinguished from other compounds that might have the same mass.
|
|
|
|
What are purines made from?
|
3 amino acids (aspartate, glycine and glutamine)& a folate derivative
|
|
|
|
How are they made (pathway)?
|
PRPP + Glutamine -->(Common pathway) --> IMP (o IMP can then be turned into AMP & GMP)
|
|
|
|
How/where is this pathway regulated?
|
Feedback inhibition
|
|
|
|
What drug targets the purine biosynthesis pathway and how does it work?
|
6-mercaptopurine
-Activated by HGPRT to form 6-MP nucleotide and acts as fake purine nucleotide -Results in feedback inhibition of all three critical steps (1st of common & 1st of both branches) |
|
|
|
Where do all the ingredients of purine nucleotide biosynthesis come from?
|
-The sugar is from <--PRPP<-- ribose-5-phosphate <-- pentose phosphate pathway
-Nitrogenous base <-- glutamine <-- glutamate -Nitrogenous base <--N10-formyl-tetrahydrofolate <-- folic acid -Nitrogenous base <-- glycine <-- serine <-- glycolysis -Phosphates are put on by specific kinases |
|
|
|
What are pyrimidines made from?
|
carbamoyl phosphate & aspartate
|
|
|
|
How are pyrimidines made?
|
-Glutamine + stuff --> carbamoyl phosphate --> orotate --> OMP --> UMP
-UMP --> UDP --> UTP -UTP can be turned into CTP in one step -dTTP is a little more complicated: UDP --> dUDP --> dUTP --> dUMP --> dTMP --> dTTP |
|
|
|
What step(s) in pyrimidines synthesis can be screwed up in disease?
|
mutation of enzyme for orotate -->UMP = orotic aciduria
-Build up orotate => excrete tons of orotic acid in urine -Bladder stones, mild mental retardation, megaloblastic anemia -Patients need to eat pyrimidines ‘cause they can’t make ‘em! -Rx: oral uridine |
|
|
|
Where do all the ingredients of pyrimidine nucleotide biosynthesis come from?
|
-The sugar is from <--PRPP
-Nitrogenous base <-- carbamoyl phosphate + aspartate -Phosphates put on by specific kinases |
|
|
|
What is the substrate for the enzyme thymidylate synthetase
|
dUMP
|
|
|
|
Both CDP and UDP can be precursors of dUTP. T/F
|
True
|
|
|
|
Describe how you can get dTMP from CDP and UDP
|
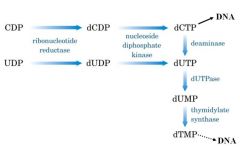
|
|
|
|
What two enzymes must work together in Thymidine synthesis?
|
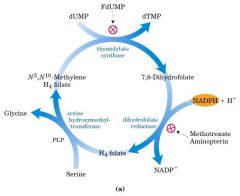
Thymidylate synthase + Dihydrofolate reductase (DHFR
|
|
|
|
What drugs inhibit the synthesis of dTMP?
|
-5-fluorouracil: converted to fDUMP and used as fake substrate that inhibits thymidylate synthase
-Methotrexate: inhibits DHFR |
|
|
|
What is another way to generate dTMP?
|
Thymidine kinase (TK) is an enzyme that adds a phosphate to deoxythymidine to make dTMP
Deoxythymidine is from <--degradation of DNA from <-- food & other dead cells [this is PYRIMIDINE SALVAGE/recycling] -The dUMP route (above) is used by cells normally and it’s making enough dTMP for DNA repair/maintenance -But when cells divide and DNA replication is needed, TK is expressed to increase dTMP supply -After division, return to normal and get rid of TK (known as a “salvage enzyme”) -We have our own TK, but so do viruses! |
|
|
|
What drugs target viral TKs?
|
-Acyclovir: for Herpes (can take systemically b/c specific for viral TK)
-IUdR (iododeoxyuridine): for corneal Herpes (put topically b/c affects human TK) -AZT (zidovudine) & ddI (dideoxyinosine): for HIV (can be used by human TK, but preferred by HIV TK) |
|
|
|
you need to remove the 2’ OH group on sugar ribonucleotides to generate the deoxyribonucleotides needed for DNA synthesis (dATP,dGTP, dTTP, dCTP). T/F
|
True
|
|
|
|
What enzyme works on all four ribonucleotides to pluck off the –OH group?
|
Ribonucleotide reductase
|
|
|
|
What disease is associated with the dysregulation of Adenosine DeAminase enzyme ?
|
ADA-SCID
|
|
|
|
What is different about adenosine & guanosine breakdown?
|
-Adenosine gets deaminated to inosine
-Guanosine gets deribosylated to its free base form guanine |
|
|
|
What is the intermediate product of purine catabolism?
|
Xanthine
|
|
|
|
What is the end product that is excreted out of the body after purine catabolism?
|
Uric acid
|
|
|
|
What disease is due to excess uric acid accumulating in the body after after purine catabolism?
|
Gout
|
|
|
|
Why is the buildup of uric acid a problem in the body (why does it cause gout)?
|
it’s insoluble, so forms crystals in joints (especially where it’s cooler temperate, like extremities) = ouch! pain in joints of fingers/toes
-Hyperuricemia = increased blood levels of uric acid -Either due to (a) more purine degradation or (b) decreased uric acid clearance -80% of gout cases due to problems in renal excretion -Rx: Allopurinol is xanthine oxidase inhibitor that prevents conversion of xanthine to uric acid -Rx: avoid ingestion of items rich in purines (that includes: alcohol ) |
|
|
|
What is the role of purine catabolism in reperfusion injury?
|
-Ischemia (lack of O2) --> direct cell injury or indirect injury upon reperfusion (blood returns)
-Reperfusion injury seen in myocardial infarctions, strokes , tissue transplantations -During ischemia, ATP is not regenerated -Xanthine oxidase enzyme catalyzes oxidation of hypoxanthine to xanthine and then also catalyzes oxidation of xanthine to uric acid -Ischemia causes the cleavage of xanthine oxidase to a form that produces superoxide free radicals --> oxidative damage -Reperfusion = blood supply returns after period of ischemia -Reperfusion injury = absence of O2 and nutrients from blood creates condition in which restoration of circulation results in inflammation and oxidative damage through induction of oxidative stress |
|
|
|
What cells utilize purine salvage pathways?
|
Actively dividing cells
|
|
|
|
What are the exact substrates that are salvaged?
|
Free purine bases, which include:
-Hypoxanthine …salvaged to --> IMP -Guanine …salvaged to --> GMP -Adenine …salvaged to --> AMP |
|
|
|
What are the purine salvage enzymes?
|
Hypoxanthine & guanine use HGPRT
-Notice how PRPP is used here (remember this guy from purine & pyrimidine synthesis?) -Adenine uses APRT (adenine phosphoribosyl transferase) |
|
|
|
What are sources of xanthine?
|
Adenosine & guanosine nucleotides
|
|
|
|
What disease is associated with lack of HGPRT?
|
Lesch-Nyhan syndrome:
-X-linked point mutation (one single nucleotide difference!) -Results in: -Hyperuricemia--> Gout -Mental retardation -Cerebral palsy -Self mutilation -HGPRT deficiency --> -increased turnover of purines (since salvage is blocked) -unused PRPP stimulates increased synthesis of purines -allopurinol helps reduce the hyperuricemia & gout pain, but not the neurological Sx |
|
|
|
What intermediate in thymine catabolism is proportional to the amount of tissue damage?
|
β-aminoisobutyric acid (AIBA)
|
|
|
|
What two steps are needed to go from free base to usable nucleotide form?
|
-Attachment of base to ribose
-Phosphorylation of nucleoside to monosphophate nucleotide |
|

