![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
116 Cards in this Set
- Front
- Back
|
The four key biomolecules of life:
|
proteins
nucleic acids lipids carbohydrates |
|
|
_____ allow for dynamic interactions and permit energy and information to move about the cell and organism.
|
Weak bonds
|
|
|
_______ form the basis of biochemistry and life itself
|
Transient chemical interactions
|
|
|
_____ is the solvent of life
|
Water
|
|
|
Our experience of life happens at a distance of _____ angstroms
|
4
|
|
|
What is the typical length of a non covalent bond?
|
4 angstroms
|
|
|
Weak, reversible but essential interactions
|
transient interactions
|
|
|
Describe Brownian Motion:
|
The water and gas molecules of the environment are bouncing randomly about at a rate determined only by the temperature. When molecules collide with pollen granules or dust motes, these molecules then themselves move randomly.
|
|
|
How many amino acids are protiens typically made of.
|
50-300
|
|
|
what two components of amino acids are hydrogen bonded to form a secondary structure
|
NH
CO |
|
|
what is the highest level of structure that an individual polypeptide can attain
|
tertiary
|
|
|
the linkage joining amino acids in a protein is_____
|
peptide bond or amide bond
|
|
|
each amino acid unit in a polypeptide is called what
|
residue
|
|
|
which terminal is the begging of the amino acid and which is the last
|
N-terminal
C-terminal |
|
|
a polypeptide chain consists of a regularly repeating part called _____
|
Main chain or backbone
|
|
|
the variable part of a polypeptide chain is called _____
|
side chain
|
|
|
all residues contain these two groups with the exception of _____
|
C==O
N-H with the exception of proline |
|
|
in a residue which group is a good hydrogen bond acceptor and which is a good hydrogen bond donor
|
the C==O is the good acceptor and the N-H is the good donor
|
|
|
Most natural polypeptide chains contain between 50 and 2000 amino acid residues and are commonly referred to as _____
|
Proteins
|
|
|
What is the largest protein known and how many amino acids does it contain
|
the muscle protein titian and it consists of 27,000 amino acids
|
|
|
peptides made of small numbers of amino acids are called_____
|
oligopeptides or peptides
|
|
|
The mean molecular weight of an amino acid residue is ______
|
110g mol-1
|
|
|
a dalton is a unit of mass equal to_____
|
a hydrogen atom
|
|
|
a protein with a molecular weight of 50,000g mol-1 has a mass equal to
|
50,000 daltons
|
|
|
how many kd is 50,000 daltons
|
50
|
|
|
The most common cross-links are____
|
disulfide bonds
|
|
|
the resulting unit of two linked cysteines is called____
|
cystine
|
|
|
what was the first discovery that showed for the first time that a protein has a precisely defined amino acid sequence?
|
the amino acid sequence of insulin
|
|
|
in the body are amino acids L amino acids or R amino acids
|
L
|
|
|
what is the amino terminus of the tripeptide Gly-Ala-Asp
|
Gly
Glycine |
|
|
What is the approximate molecular weight of a protein composed of 300 amino acids?
|
33,000
|
|
|
How many amino acids are in a protein with a molecular weight of 110,000?
|
1,000
|
|
|
what determines the 3-D structures of proteins?
|
The amino acid sequence
|
|
|
what are two examples of diseases resulting from a change in a single amino acid within a protein
|
sickle-cell anemia
cystic fibrosis |
|
|
what is the geometry of a peptide bond
|
planer
|
|
|
for a pair of amino acids linked by a peptide bond how many atoms lie in the same plane? What are they?
|
6 atoms
the alpha-carbon atom and the CO group of the first amino acid and the NH group and the alpha-carbon atom |
|
|
can rotation occur around a peptide bond... Why or Why not?
|
no because the peptide bond has considerable double-bond character because of resonance.
|
|
|
the C---N bond distance in a peptide bond is _____?
What is the normal C---N single bond distance? What is the normal C===N distance? |
1.32 Angstroms
1.45 Angstroms 1.27 Angstroms |
|
|
are peptide bonds charged or uncharged and why is this important?
|
Uncharged
globular structures are not inhibited by a charge repulsion |
|
|
Give the bond lengths within a peptide unit:
C1alpha - C C==O C-N peptide bond N-H N-C2alpha |
C1alpha - C 1.51 Angstroms
C==O 1.24 Angstroms C-N peptide bond 1.32 Angstroms N-H 1.0 Angstroms N-C2alpha 1.45 Angstroms |
|
|
almost all peptide bonds in proteins are______(cis/trans)
Why? |
Trans
steric hindrance |
|
|
the _________ about two bonds of each amino acid allows proteins to fold in many different ways
|
freedom of rotation
|
|
|
in an amino acid backbone, which bond has free rotation?
|
amino group and C2 alpha carbon
|
|
|
What is the name of the angles in a peptide chain between the N-Calpha and the C-carboxyl Carbon?
|
torsion angles
N-C alpha phi C-C carboxyl psi |
|
|
What are the two periodic structures amino acids can fold into?
|
alpha helix
beta pleated shet |
|
|
How is an alpha helix stabilized?
|
hydrogen bonds between the NH and CO groups of the main chain. The CO group of each amino acid forms a hydrogen bond with the NH group of the amino acid that is situated four residues ahead in the sequence.
|
|
|
In an alpha helix, how is one residue related to the next? (term)
|
rise or translation
|
|
|
what is the rise of one residue to the next in an alpha helix?
|
1.5 Angstroms along the helix axis
|
|
|
what is the translation of one residue to the next in an alpha helix?
|
1.5 Angstroms along the helix axis.
|
|
|
what is the rotation of one residue in an alpha helix to the next?
|
100 degrees, giving 3.6 per turn
|
|
|
How many residues are in a full turn of an alpha helix?
|
3.6 (100 degrees each)
|
|
|
Define the pitch of an alpha helix and give the length.
|
The pitch of an alpha helix is the length of one complete turn of the alpha helix and is the product of the translation (1.5 Angstroms) and the number of residues per turn (3.6) = 5.4 Angstroms
|
|
|
Essentially, all alpha helices found in proteins are _____ - handed
|
right
|
|
|
Which amino acids are not readily accommodated in an alpha helix and why?
|
Branching at the beta carbon atom of valine, threonine, and isoleucine destabilizes alpha helices because of steric hinderance.
Serine, aspartate, and asparagine disrupt alpha helices because their side chains are hydrogen-bond donors or acceptors in close proximity to the main chain where they compete for NH and CO groups Proline breaks a helix because it's ring structure involves the N which should be a part of the backbone. |
|
|
A beta sheet is composed of two or more polypeptide chains called _____
|
beta strands
|
|
|
The distance between adjacent amino acids in a beta chain is _____
|
3.5 Angstroms
|
|
|
Do the side chains of a beta sheet point in the same or opposite directions?
|
opposite directions
|
|
|
If two adjacent chains in a beta sheet run in opposite directions, it is called _____. If two adjacent chains in a beta sheet run in the same direction, it is called _____
|
antiparallel beta sheet;
parallel beta sheet |
|
|
With antiparallel/parallel beta sheets, which one has the components of one amino acid hydrogen bonding with the components of only one other amino acid?
|
Antiparallel;
parallel: one amino acid hydrogen bonds with two other amino acids |
|
|
Are turns and loops situated on the inside or surface of a beta sheet or alpha helix?
|
surface
they interact with other molecules in solution |
|
|
Are turns and loops are usually composed of amino acids with (hydrophobic/hydrophilic) R groups?
|
Hydrophilic
|
|
|
Special types of helices are present in two common fibrous proteins:
|
alpha-keratin;
collagen |
|
|
____ is the primary component of wool and hair
|
alpha-keratin
|
|
|
alpha-keratin is composed to two (right/left)-handed alpha helices to create a type of (right/left)-handed superhelix called a _______.
|
right handed;
left handed; coiled coil protein |
|
|
Intermediate filaments and the muscle proteins myosin and tropomyosin are made of _____
|
coiled coil proteins
|
|
|
The two helices in alpha keratin are cross-linked by:
|
van der Waals forces and ionic interactions or by disulfide bonds formed by neighboring cysteine residues
|
|
|
What is the most abundant mammalian protein?
|
collagen
|
|
|
In what type of helix is glycine every third residue?
|
collagen
|
|
|
Every third residue in collagen is _____
|
glycine
|
|
|
What is absent in a collagen helix?
|
hydrogen bonds
|
|
|
How are the helices in collagen stabilized?
|
steric repulsion of the pyrrolidine rings of the proline residues
|
|
|
How many helix strands are in collagen?
|
Three
|
|
|
In a superhelical cable of collagen, how are the different strands of helices stabilized?
|
hydrogen bonds between the NH groups of glycine residues and the CO groups of residues on the other chains.
|
|
|
In collagen, why does glycine have to appear every third residue?
|
because the center helix is very crowded and glycine is a small amino acid.
|
|
|
The only residue that can fit in an interior position of a superhelical cable is ____
|
glycine
|
|
|
What is the clinical name of brittle bone disease?
|
osteogenesis imperfecta
|
|
|
What is the pathology of osteogenesis imperfect?
|
The inner glycine residue is replaced by other amino acids
|
|
|
The accumulation of defective collagen results in _____
|
cell death
|
|
|
Vitmin C is required for the formation of stable collagen fibers because it assists in the formation of _____ from _____ in the (1st, 2nd proline)
|
hydroxyproline; proline; 2nd
|
|
|
What are the symptoms of scurvy? What causes it?
|
skin lesions, blood-vessel fragility
Caused by less stable collagen due to a lack of hydroxyproline due to lack of vitamin C |
|
|
_____ refers to the spatial arrangement of amino acid residues that are far apart in the sequence and to the pattern of disulfide bonds.
|
tertiary structure
|
|
|
Tertiary structure is the result of interactions between _____
|
R groups of the polypeptide chain
|
|
|
Are globular proteins water soluble or lipid soluble?
|
Water soluble
|
|
|
How many amino acids are in myoglobin?
|
153 amino acids
|
|
|
What protein performs most of the chemical transactions in the cell?
|
globular proteins
|
|
|
Where is myoglobulin found?
|
predominantly in the heart and skeletal muscle
|
|
|
What does moglobulin do?
|
It is an oxygen-ginding protein and it appears to facilitate the diffusion of oxygen from the blood to the mitochondria, the primary site of oxygen utilization in the cell.
|
|
|
What is heme?
|
a prosthetic (helper) group containing an iron atom
|
|
|
Is myoglobin an extremely compact molecule?
|
yes
|
|
|
What are the overall dimensions of a myoglobin protein??
|
45 x 35 x 25 Angstroms
|
|
|
how many alpha helices does myoglobin have?
|
8
|
|
|
What are the only polar residues on the interior of a myoglobin protein?
|
Two histidine residues
|
|
|
What do the two histidine amino acids on the interior of a myoglobin protein do?
|
Play critical roles in binding the heme iron and oxygen.
|
|
|
Does the outside of a myoglobin consist of only polar, only nonpolar, or both types of residues?
|
both, which can render the molecule water soluble
|
|
|
Certain combinations of secondary structure are present in many proteins and frequently exhibit similar functions. These combinations are called _____
|
motifs or supersecondary structures
|
|
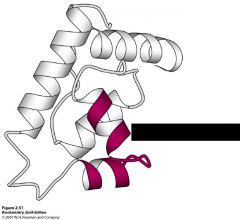
What motif is this?
|
helix-turn-helix
|
|
|
______ refers to the arrangement of subunits and the nature of their interactions.
|
Quaternary structure
|
|
|
What is the simplest sort of quaternary structure?
|
dimers
|
|
|
What is a dimer?
|
the simplest form of quaternary structure consisting of two identical subunits.
|
|
|
How many alpha and how many beta subunits does human hemoglobin have?
|
2 alpha
2 beta |
|
|
Human hemoglobin exists as ______ tetramer
|
alpha2beta2 tetramer
|
|
|
the enormous difference between calculated and actual folding times in a protein is called _____
|
Levinthal's paradox
|
|
|
What are IUP's?
|
Intrinsically Unstructured Proteins
|
|
|
IUP's are important in _____ and _____
|
Signaling and regulatory pathways
|
|
|
What is the general name for diseases that occur due to protein aggregate formation from misfolded proteins?
|
Amyloidoses
|
|
|
Which amino acids are hydrophobic?
|
(9)
Glycine (Gly, G) Alanine (Ala, A) Valine (val, V) Leucine (Leu, L) Isoleucine (Ile, I Methionine (Met, M) Proline (Pro, P) Phenylalanine (Phe, F) Tryptophan (Trp, W) |
|
|
Which amino acids are polar but not charged?
|
(6)
Serine (Ser, S) Threonine (Thr, T) Tyrosine (Tyr, Y) Cysteine (Cys, C) Asparagine (Asn, N) Glutamine (Gln, Q) |
|
|
Which three amino acids contain hydroxyl (OH-) groups?
|
Serine (Ser, S), threonine (Thr, T), tyrosine (tyr, Y)
|
|
|
This is a version of alanine (Ala, A) with a hydroxyl group attached to it:
|
Serine (Ser, S)
|
|
|
These two amino acids are similar but one contains a sulfhydryl and one replaces the sulfhydryl with a hydroxy group.
|
Cysteine (sulfhydryl) Cys, C
Serine (hydroxyl) Ser, S |
|
|
Which two amino acids are polar and contain a carboxylamino group?
|
Asparagine (Asn, N) and Glutamine (Gln, Q)
|
|
|
Positively charged amino acids are (hydrophobic/hydrophilic)
|
Hydrophilic
|
|
|
What are the hydrophilic amino acids?
|
(3)
Lysine (Lys, K) Arginine (Arg, R) Histidine (His, H) |
|
|
These two amino acids have long side chains that are positively charged at the end
|
Lysine (Lys, K)
Arginine (Arg, R) |
|
|
This amino acid contains an imidazole group.
|
Histidine (His, H)
|
|
|
What amino acids are negatively charged?
|
Aspartate (Asp, D)
Glutamate (Glu, E) |

