![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
69 Cards in this Set
- Front
- Back
|
What are the hydrophobic aliphatic amino acids?
|
P. GALVIM:
Proline, Glycine, Alanine, Leucine, Valine, Isoleucine, Methionine. *What kind of amino acids are these? |
|
|
What are the hydrophobic aromatic amino acids?
|
TPT: Tryptophan, Phenylalanine, Tyrosine
*What kind of amino acids are these? |
|
|
What are the hydrophilic, uncharged amino acids?
|
GAsp STC
Glutamine, Asparginine, Serine, Threonine, Cystein *What kind of amino acids are these? |
|
|
What are the hydrophilic, charged amino acids?
|
LaHag:
Lysine, Arginine, Histidine, Aspartate, Glutamate *What kind of amino acids are these? |
|
|
How can there be more than 20 amino acids?
|
Post-translational modification.
|
|
|
What determines the 3D structure of a protein?
|
Its primary structure.
|
|
|
What is a primary structure?
|
It is the linear amino acid sequence itself.
|
|
|
Define secondary structure.
|
Secondary structure is defined by patterns of hydrogen bonds between backbone amide and carboxyl groups. They form spontaneously with hydrophobic aa's within the core and hydrophilic aa's on the surface.
|
|
|
Secondary folding typically follows a hierarchy - describe that.
|
Usually, H-bonds between peptide bond and carbonyl (C=O) groups and peptide bond amide hydrogens (>N-H) form first.
|
|
|
Alpha-helices, parallel and anti-parellel B-pleated sheets are examples of what kind of structures?
|
Secondary
|
|
|
Define tertiary structure.
|
The folding pattern of the secondary structure into a three-dimensional conformation
|
|
|
Define quaternary structure.
|
The structure formed by the noncovalent interaction of two or more macromolecules.
|
|
|
What is the major structural difference between globular proteins and structural proteins?
|
Globular are more spherical or ellipsoidal vs structural that are elongated and very stable.
|
|
|
What two structural proteins have unusual crosslinks that allow it to stretch without breaking?
|
Alpha-keratin and elastin
|
|
|
Why do proteins typcially spontaneously fold into a 3D structure?
|
To achieve the lowest free energy of all possible folding patterns.
|
|
|
Except for proline, each aa has three features bonded to the alpha-carbon atom. What are these and what is proline missing?
|
A carboxyl group, an amino group, and a distinctive side chain. Proline is missing the typical amino group that is replaced by an imino group.
|
|
|
Draw an aa backbone
|
H3N-C-COOH - with an H above a-C and a R below a-C
|
|
|
What makes an aa chiral? What is the only one that is not?
|
Chiral if 4 different groups attached to a-C. Glycine has two H- and thus not chiral
|
|
|
AA exist as mirror image form known as _____ that are termed ___ and ____
|
Enantiomers termed D- (dextro; right) and L- (levo; left). (Note: Even though L- and D- rotate light in opposite directions, you can't predict which way they'll turn by the L- or D- designation alone. L- and D- refer to the actual structure only.)
|
|
|
Which enantiomeric configuration do all mammalian protein use? What is it in bacteria that penicillin targes?
|
L-config. Bacteria use D-amino acids in cell wall peptidoglycans so using abx that target D-aa interfer with only bacteria cell wall.
|
|
|
How do you determine the chirality of aa's?
|
H = thumb. NH3 is given highest priority = 1; COOH is given second; R is given 3rd. Determine which hand follows priority in order as you turn it.
|
|
|
The three letter designation for the AA's is pretty straight forward. The three that are a little challenging are Aspartate, Asparagine, and Glutamine. What are the?
|
Asn, Gln,
|
|
|
Describe the aromatic amino acids side chain: Three things:
1. Aromatic derivation 2. Spacer 3. Absorbance |
1. Aromatic side chain is derived from benzene (Phe Tyr) or indol (Trp).
2. Each contains a methylene spacer (-CH2-) bn the aromatic ring and a-C to minimize the steric repulsion bn the ring and polypeptide chain backbone. 3. Aromatic side chains have UV absorbance structure that allow protein concentrations to be estimated via 280nm absorbance. |
|
|
What makes tyrosine an important regulator?
|
It has an OH- group on its ring that can be phosphorylated and serve as an important responder to growth factors. Several cancer therapies are inhibitors of tyrosine kinases.
|
|
|
What do serine and threonine possess that make them play important roles in regulation?
|
-OH groups.
|
|
|
Asn and Gln both carry -NH2 groups in their side chains - are they ever protonated?
|
No! The are not ionic and amides (have adjacent carbonyl), not amines.
|
|
|
Where do charged aa's carry their charge?
|
On their side chains
|
|
|
Charged aa's have side chains that can carry either a positive or negative charge. Which carry + and which carry -?
|
Those with -NH2 can add an H+ to make them positive and those with -COOH can lose an H+ to become negative.
|
|
|
Which charged polar aa's can carry a pos charge? Which neg charge?
|
LAH - Lysine, Arginine, Histidine (LArgH)
GA - Glutamate, Aspartate (GAsp) |
|
|
Define pKa
|
The pH at which a compound is 50% protonated and 50% unprotonated.
|
|
|
What is the Henderson-Hasselbalch equation?
|
pH = pKa + log [A]/[HA]
|
|
|
The pKa of Aspartic acid is 4.1. If the pH of the solution is 5.1, what is the ratio of the concentration of the deprotonated form to the protonated form?
|
10:1. I.e. there's a lot more deprotonated than protonated aa around.
|
|
|
Define isoelectric point.
|
The pH where the net charge of a protein = 0
|
|
|
Alanine has two pKa's:
1. pKa = 2.3 2. pKa = 9.1 What is the charge of the molecule at pH<2? pH 6? pH>10? |
pH 2 = positively charged.
pH 6 = neutral charge pH >10 = negatively charged |
|
|
In regard to titration, at what points are aa's buffered?
|
When the pH is near the pKa - it takes more equivalents to change the pH then when not.
|
|
|
How do you calculate the pI?
|
pI = (pKa1 + pKa2)/2
|
|
|
What are the two aa's that contain sulfur?
|
Methionine and cysteine
|
|
|
AAs of proteins are joined by peptide bonds between the ____ group of one aa and the _____ of the next. Proteins are synthesized on ribosomes from ?-terminal to ?-terminal and by convention drawn with ?-to the left.
|
Carboxyl group, Amino group.
Synthesized from N-term to C-term with N-term to the left. |
|
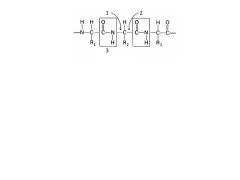
Label these
|
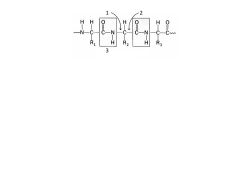
1. Phi
2. Psi 3. Rigid unit |
|
|
Why is the rigid unit of a peptide bind mostly fixed?
|
Bc the carbonyl oxygen and amide nitrogen share electrons to create resonance (electrons spend more time around carbonyl ~60%). The resonance/double bond character make it difficult to swivel.
|
|
|
Alpha-helixes are __1__-handed and contain __2__ residues per turn. They are stabilized by H-bonds bn the __3___ of amino acid i and the __4__ of amino acid i+4.
|
1. Right
2. 3.6 3. Carbonyl 4. Amide proton |
|
|
How are B-pleaded sheets similar in terms of their H-bonding? How are they different?
|
B-sheets also have H-bonds between carbonyl oxygen atoms and amide protons BUT they're between non-contiguous parts of the polypeptide chain
|
|
|
Are peptide bonds polar or nonpolar?
|
They are polar.
|
|
|
If peptide bonds are polar, how/why are they readily found in the hydrophobic environment inside proteins?
|
Because they can H-bond with each other rather than water.
|
|
|
Why are proline residues typically found at the end of Alpha helices and B-pleated sheets?
|
Bc the ring structure in Proline prevents it from forming H-bonds required in Alpha and Beta structures.
|
|
|
Define super-secondary structures.
|
The arrangement of alpha-helices or beta-strands in a protein sequence into discrete folded structures: e.g., beta-barrels, or beta-alpha-beta-motifs.
|
|
|
Define domains
|
The smallest thermodynamically stable units of protein structure.
|
|
|
Why are proline residues typically found at the end of Alpha helices and B-pleated sheets?
|
Bc the ring structure in Proline prevents it from forming H-bonds required in Alpha and Beta structures.
|
|
|
Why are proline residues typically found at the end of Alpha helices and B-pleated sheets?
|
Bc the ring structure in Proline prevents it from forming H-bonds required in Alpha and Beta structures.
|
|
|
Define super-secondary structures.
|
The arrangement of alpha-helices or beta-strands in a protein sequence into discrete folded structures: e.g., beta-barrels, or beta-alpha-beta-motifs.
|
|
|
Define super-secondary structures.
|
The arrangement of alpha-helices or beta-strands in a protein sequence into discrete folded structures: e.g., beta-barrels, or beta-alpha-beta-motifs.
|
|
|
Define domains
|
A thermodynamically stable, discrete portion of a protein with its own function. The combination of domains in a single protein determines its overall function.
|
|
|
Define domains
|
A thermodynamically stable, discrete portion of a protein with its own function. The combination of domains in a single protein determines its overall function.
|
|
|
Describe the structure of a-Keratin
|
Consists of two right-handed a-helices that are intertwined in a left-handed super-coil called an a-coil
|
|
|
What is a-Keratin the primary component of?
|
Hair and nails
|
|
|
What makes a-Keratin stretchable? What makes it less so as in nails and horns?
|
Disulfide allow it to stretch but return to normal shape. A much higher number of disulfid cross-links makes nails and horns more rigid.
|
|
|
Describe the structure of collagen. Which way do individual helices turn? Which way does the complex helix turn?
|
It is a long, rigid structure in which three polypeptides that are wound around each other in a rope-like triple helix. Individual turns left. Complex turns right.
|
|
|
What stabilizes the a-Keratin structure?
|
H-bond, ionic bonds, hydrophobic interactions, and disulfide bonds
|
|
|
What is the generic aa sequence of collagen?
|
Gly-X-Y where Glycine repeats every third residue and X is frequently a proline and Y is either hydroxyproline or hydroxylysine.
|
|
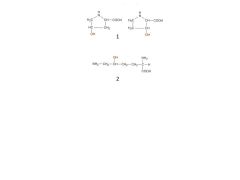
What are these compounds? Why are they important?
|
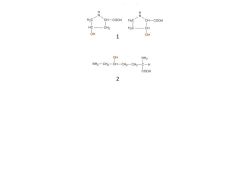
1. Hydroxyproline
2. Hydroxylysine They are made by post-translational hyroxylation rxns that require vitamin C. If no vitamin C, this rxn does not occur, decreasing the tensile strength of collagen and causing scurvy |
|
|
Regarding collagen assembly, what steps occur inside the cell to make procollagen? Then what happens?
|
Procollagen is synthesised, post-translational modified, and triple helix assembly inside. Then pro- is secreted where N- and C-terminal ends are cleaved and TROPOCOLLAGEN self-assembles into fibrils with subsequent cross-linking to form mature fibers.
|
|
|
Elastin has what kind of characteristic? Where is it typically found?
|
Rubber-like. Found in lungs, large arterial walls, elastic ligaments.
|
|
|
Elastin is composed mainly of small, nonpolar aa's (1,2,3) and rich in 4, 5. IT cross-links with 6 residues of neighboring peoplypeptides to produce an extensively interconnected compound.
|
1. Valine
2. Alanine 3. Glycine 4. Proline 5. Lysine |
|
|
What does alpha-antitrypsin do?
|
It inhibits elastase.
|
|
|
1. Genetically, how can a-AT be a problem?
2. Envrionmentally, how can a-AT be a problem? |
1. A genetic defect can prevent a-AT from being released from the cell - leaving elastase free to over-degrade elastin
2. Environmental factors such as smoking cause a methionine group in a-AT to be oxidized - preventing it from working properly. |
|
|
What is elastase, from what is it released, and what can happen if not controlled?
|
It is an enzyme released by neutrophils and functions to degrade elastin. If elastase is not controlled, it will degrade alveolar epithelium and may lead to emphysema.
|
|
|
Describe the shape of a titration curve at the following points:
pKa pI buffer zone |
pKa = relatively vertical
pI = relatively horizontal buffer zone = relatively vertical |
|
|
Describe the turns for
1. Alpha-helix 2. Collagen fibers 3. Alpha-keratin fibers. |
1. Rt turn helices
2. Three Lt-turn helices to make one Rt super helix 3. Two Rt- turn helices to make one Lt turn super helix. |
|
|
Review proteases and peptidases mechanism. How Glu72, His69, His196. from p35 study with JMP
|
Review proteases and peptidases mechanism. How Glu72, His69, His196. from p35 study with JMP
|

