![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
27 Cards in this Set
- Front
- Back
|
purpose of the citrate shuttle |
FA synthesis occurs in the cytosol, but building blocks for FA synthesis (acetylCoA) are present in MT matrix -acetylCoA needs to be tranpsorted into the cytosol to undergo FA synthesis |
|
|
steps of acetylCoA using citrate shuttle |
1. citrate synthase condenses OAA w acetylCoA to make citrate in matrix 2. citrate is transported through the citrate transporter (located on the inner MT matrix) 3. when citrate enters the cytosol it is cleaved into acetylCoA and OAA by citrate lyase -this step uses -1ATP
there is no transporter to allow OAA back into matrix 4. cytosolic malate dehydrogenase reduces OAA to malate 5. malate can re-enter matrix or be oxidized to pyruvate (via malic enzyme) 6. pyruvate is carboxylated (via pyruvate carboxylase) to re-form OAA in matrix |
|
|
what are the 2 main sources of reducing equivalent NADPH required for FA synthesis |
1. malic enzyme (1 NADPH)
2. pentose phosphate pathway (2 NADPH) |
|
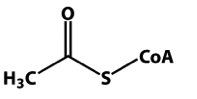
|
acetyl CoA |
|
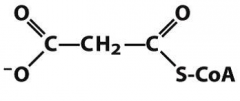
|
malonylCoA |
|
|
draw acetylCoA |
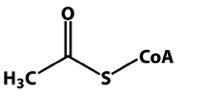
|
|
|
draw malonylCoA |
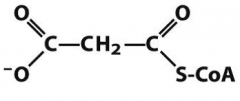
|
|
|
synthesis of malonylCoA |
enzyme acetylCoA carboxylase (ACC) producse malonylCoA from acetylCoA
-HCO3- is used to provide the additional carbon to acetylCoA -this requires -1ATP -this step is irreversible
this is the committed step in FA synthesis! |
|
|
FAS I |
fatty acid synthase I consists of a single multifunctional polypeptide chain -2 of these chains come together to form a homodimer -has 7 domains -2 domains are: 1. beta-ketoacyl-ACP synthase (KS) domain 2. Acyl Carrier Protein (ACP) |
|
|
KS domain of FAS |
beta-ketoacyl-ACP synthase
-contains a thiol (-SH) group used to bind acetylCoA and malonylCoA and when they are hydrolyzed off these thiol groups, the rxn is highly exergonic |
|
|
ACP domain of FAS |
aceyl carrier protein
-contains a thiol (-SH) group used to bind acetylCoA and malonylCoA and when they are hydrolyzed off these thiol groups, the rxn is highly exergonic |
|
|
4 steps to FA synthesis |
(2 preliminary steps) 1. Condensation 2. Reduction 3. Dehydration 4. Reduction |
|
|
preliminary step 1 of FA synthesis |
acetyl group (from acetylCoA) is transferred onto the thiol group of ACP 1 CoA is lost
the acetyl group is then transferred from ACP onto KS |
|
|
preliminary step 2 of FA synthesis |
the malonyl group is transferred from malonylCoA to the thiol group on ACP
|
|
|
step 1 FA synthesis condensation |
KS performs a condesnation rxn w the acetyl and malonyl groups (and now attached to only ACP) during this step, decarboxylation of the malonyl occurs (CO2 released)
the loss of CO2 makes the condensation rxn exergonic (favourable) |
|
|
step 2 FA synthesis Reduction |
the carbonyl at C3 now undergoes a reduction NADPH is used this is catalyzed by KR
the beta-carbon is in D-configuration |
|
|
step 3 FA synthesis Dehydration
|
an H2O molecule is removed (remove OH group and form double bond between C2 and C3)
|
|
|
step 4 FA synthesis Reduction |
the trans double bond is reduced (and saturated) by enzyme ER NADPH is used
C4:0-ACP is formed as the final product after the first round of FA synthesis |
|
|
step 5 FA synthesis |
C4:0-ACP is transferred onto the -SH group of KS in preparation for the next round of FA synthesis |
|
|
step 6 FA synthesis |
another malonylCoA "recharges" ACP catalyzed by MAT |
|
|
enzyme TE |
catalyzes rxn of using a water molecule to release the finished product from FAS in FA syntehsis |
|
|
fatty acid elongation system |
process that makes palmitate (C16:0 - product of FAS I) into longer-chain FAs -present on cytosolic face of smooth ER -elongations occur by adding 2-C units in the form of malonyl CoA |
|
|
fatty acyl-CoA desaturase |
enzyme used to introduce double bonds in the saturated FA chains |
|
|
essential FAs in mammal diet |
mammals are unable to produce double bonds beyond C-9 in the FA chain
C18:2 cis-Δ9,12 C18:3 cis-Δ9,12,15 C20:5 cis-Δ5,8,11,14,17 C22:6 cis-Δ4,7,10,13,16,19 |
|
|
regulation of ACC |
ACC plays role in β-ox and FA synthesis general rule: ACC activated in times of plenty and deactivated when energy is required
citrate diverts cell metab from consumption of fuel to storage of fuel as FAs (citrate shuttle - citrate moved out of MT matrix into cytosol when high [acetylCoA], high [ATP]
palmitoyl-CoA (C16:0-CoA) - palmitate is end product, when attached to CoA, inactivates ACC |
|
|
hormonal control of ACC |
1. when blood glucose levels are high, insulin is secreted from pancreas 2. insulin-dependent phosphatase dephosphorylates ACC, activating ACC 3. when ACC is activated, acetyl CoA is carboxylated to form malonyl CoA 4. malonylCoA inhibits carnitine acyltransferase I (CAT I) (this prevents additional FAs from being oxidized - inhibits transport into MT matrix) 5. when blood glucose levels are low, glucagon secreted by pancreas 6. activate protein kinase A (PKA), which phosphorylates ACC, inactivating it 7. FAs allowed to enter MT matrix 8. FAs will be used as fuel source for ATP. FAs are oxidized via β-ox |
|
|
AMPK |
AMP-dependent protein kinase
acts as a fuel gauge in the cell
activated by high [AMP] inhibited by high [ATP] |

