![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
288 Cards in this Set
- Front
- Back
|
2 types of adhesion molecules.
|
1. Integrins
2. Cadherins |
|
|
What do adhesion molecules do?
|
1. Hold the cell together or to a substrate
2. Are signaling platforms |
|
|
2 places integrins bind to:
|
1. CAMs and/or
2. the EC matrix |
|
|
What must cells do to be able to grow?
|
Attach, spread, and create tension.
|
|
|
If a cell is not distorted, it can lead to...
|
apoptosis.
|
|
|
Cell adhesion molecules of the integrin family consist of...
|
18 α and 8 β subunits which form 24 known αβ-heterodimers.
|
|
|
3 domains of integrins:
|
1. large extracellular domain
2. short transmembrane domain 3. small intracellular domain |
|
|
3 things integrins are the main receptors for:
|
1. collagen
2. fibronectin 3. laminin. |
|
|
What does integrin activation do?
|
It changes affinity and signaling by opening and extending the headpiece like a balloon animal.
|
|
|
What does fibronectin do to integrins?
|
It's an extracellular protein that links integrins to the extracellular matrix.
|
|
|
2 types of molecules integrins bind at the surface:
|
1. fibronectin
2. cell surface proteins |
|
|
What is a component of the basal lamina?
|
fibronectin
|
|
|
2 ways that integrins are regulated:
|
1. They can change affinity due to change in conformation
2. Thy can cluster |
|
|
2 things integrins are regulated by:
|
1. inside out signaling - binding to ECM
2. outside in signaling - activation of signaling pathways. ex: Talin activation and binding |
|
|
How are integrins when they are inactivated vs. when they are activated by talin?
|
1. inactive: long, tilted, and packed transmembrane and extracellular proteins
2. active: open, straight and separated by binding to talin. |
|
|
Where are integrins located in relation to actin?
|
They are at the site where the focal adhesions meet the cytoskeleton.
|
|
|
What was an early insight into integrin signaling?
|
One of the earliest insights into the possibility of integrin signaling was the observation that integrin-mediated adhesion and/or clustering led to enhanced tyrosine phosphorylation. It quickly became apparent that adhesion was activating a nonreceptor tyrosine kinase now known as focal adhesion kinase (FAK).”
|
|
|
What can FAK bind to?
|
Signaling and structural proteins: c-Src, PI-3-kinase, GRAF (a RhoGAP), paxillin, talin, and p130 Cas
|
|
|
How is FAK activated?
|
Tyrosine phosphorylation
|
|
|
What does activated FAK do?
|
It accompanies integrin-mediated adhesion
|
|
|
What happens to FAK when a cell is detached?
|
It is dephosphorylated.
|
|
|
Integrin clustering leads to...
|
a signaling complex.
|
|
|
What physical act activates integrin?
|
Stretching.
|
|
|
Clustering of integrin recruits...
|
FAK
|
|
|
FAK is...
|
a scaffold protein that recruits many other proteins.
|
|
|
FAK can activate which 2 cascades?
|
1. The MAP kinase cascade.
2. PI3 kinase cascade |
|
|
What is the MAP kinase cascade?
|
(SOS->RAS=>RAF=>MEK=>MAPK=> cJun)
=> growth + apoptosis |
|
|
What is the PI3K cascade?
|
PI3Kinase => PDK + AKT => survival
|
|
|
What are cadherins?
|
calcium dependent homotypic adhesion molecules
|
|
|
What are catenins?
|
adaptor proteins.
|
|
|
Where do you find cadherins?
|
On the exterior, connecting two cells.
|
|
|
Where do you find catenins?
|
On the interior connected to the caderins.
|
|
|
Cadherin activity influences 3 things:
|
1. cell sorting and tissue segregation
2. synaptogenesis 3. cell-on-cell locomotion during gastrulation. |
|
|
The loss of e-cadherin activity can lead to...
|
Tumor progression and invasion.
|
|
|
How is cadherin related to the neural tube?
|
During development, similar cadherins come together to create differentiation and the neural tube.
|
|
|
Cadherins make _____ between lumen.
|
an adhesion belt.
|
|
|
What do Wnt proteins bind to?
|
the receptor frizzled
|
|
|
When Wnt binds to frizzled, what happens?
|
disheviled is activated.
|
|
|
What does active disheviled bind to and what does it do?
|
It binds to axin and disrupts the Axin/GSK-3/APC complex.
|
|
|
When the Axin/GSK-3/APC complex is broken up, what happens?
|
beta-catenin goes into the nucleus and activated trxn factor TCF/LEF.
|
|
|
What does TCF/LEF do?
|
Activates Wnt target genes
|
|
|
What is colon cancer associated with?
|
The Wnt signaling pathway and a mutation/loss of APC.
|
|
|
How is disheviled activated?
|
Dunno, maybe G protein as seen in frogs.
|
|
|
Define the Wnt signaling pathway:
|
Wnt binds to frizzled -> activates disheviled -> binds to axin and disrupts axin/GSK-3/APC complex -> beta-catenin goes into nucleus and activates TCF/LEF -> activates Wnt target genes.
|
|
|
What is beta-catenin and what does it do?
|
It is in a cadherin-bound form which regulates adhesion; it's in the axin/GSK-3/APC complex.
|
|
|
2 roles of beta-catenin:
|
1. developmental signaling (Wnt)
2. Regulation of epithelial cells |
|
|
Phosphorylated beta-catenin is recognized by...
|
E3 ligase.
|
|
|
A loss in cadherins leads to an increase in...
|
free beta-catenin.
|
|
|
A mutation in APC leads to...
|
colon tumors (polyps) which leads to colon cancer.
|
|
|
How would damage to colon epithelial cells automatically trigger a repair response?
|
Perhaps the axin/GSK-3/APC complex would be broken which would lead to beta-catenin signaling.
|
|
|
What would happen to adhesion complexes if there is damage?
|
The adhesion complex would break apart and lead to signaling like the the Wnt pathway.
|
|
|
4 types of junctions:
|
1. adherens junctions (cadherins)
2. desmosomes (cadherins) 3. Hemidesmosomes (integrins) 4. tight junctions |
|
|
2 subtypes of desmosomes:
|
1. desmocollin (DSC)
2. desmoglein (DSG) |
|
|
What are desmosomes?
|
They are the entire complex of catenins and cadherins that kind of act like a button to keep cells attached.
|
|
|
What do tight junctions do?
|
Form seals between epithelial cells.
|
|
|
What are gap junctions?
|
Holes or channels between cells which can pass various molecules.
|
|
|
What are gap junctions made out of?
|
2 connexons in register which form and open channel which is about 1.5 nm in diameter.
|
|
|
Connexons are made up of ___ subunits.
|
6
|
|
|
What is the basal lamina?
|
a layer of extracellular matrix formed by epithelial cells which the epithelium sits on.
|
|
|
What is the epithelium?
|
a type of animal tissue which lines cavities and glands. Like skin.
|
|
|
How does the basal lamina work with muscle cells?
|
It surrounds the very thin muscle tissue.
|
|
|
What must cells do in order to metastasize?
|
They must get past the basement membrane.
|
|
|
What is the basement membrane?
|
The fusion of two lamina, the basal lamina and the reticular lamina which the epithelium sits on.
|
|
|
What is the chemical process used to release energy in the cell?
|
Oxidation.
|
|
|
Rate these in order from most reduced to most oxidized: formaldehyde, methane, carbon dioxide, formic acid, methanol.
|
Methane, methanol, formaldehyde, formic acid, carbon dioxide.
|
|
|
What does oxidized mean?
|
It's the loss of electrons, loss of hydrogen, or the gain of oxygen.
|
|
|
What does reduced mean?
|
The loss of oxygen, the gain of electrons, or the gain of hydrogen.
|
|
|
What is glucose oxidized into?
|
CO2 and H2O.
|
|
|
3 ways in which mitochondria are self-replicating organelles:
|
1. Has its own DNA
2. Ribosomes 3. Makes ~13 of its own proteins (most are coded by nucleus and imported into the mitochondria) |
|
|
How do you get your mitochondria?
|
They're passed down from the egg. Mitochondrial DNA represents female lineage.
|
|
|
How do mitochondria use O2?
|
They use the majority of the body's oxygen (98%) to make the majority of ATP.
|
|
|
Mitochondria regulates which molecule?
|
Calcium.
|
|
|
How are MT related to apoptosis?
|
MT undergo permeability transition and release factors that induce apoptosis like AIF, cyt c, and procaspace-3.
|
|
|
Describe the outer membrane of MT:
|
Very permeable due to channels called porins and the VDAC channel.
|
|
|
What is the VDAC channel?
|
A channel in the outer membrane of the mitochondrion which is an ion channel permeable to ATP/ADP.
|
|
|
Describe the intermembrane space of the MT:
|
The space between the inner and outer membranes contains factors that when released into the cytoplasm cause apoptosis.
This includes cytochrome C. |
|
|
The inner membrane of the MT:
|
that is very impermeable. This is
Essential to maintain coupling between pumping of protons And production of ATP (we will see this later). |
|
|
Matrix of the MT:
|
Space enclosed by the inner membrane. Houses the citric acid cycle, DNA, Ribosome and associated synthetic machinery.
|
|
|
The 4 components of the mitochondrion:
|
1. the outer membrane
2. the intermembrane space 3. the intermembrane 4. the matrix |
|
|
Why does the MT oxidize compounds?
|
Mitochondria oxidize compounds to obtain “high energy” electrons
The energy released as these electrons are transferred to oxygen drive proton pumps in the inner membrane. |
|
|
What are the products and reactants of the TCA cycle?
|
Pyruvate --> AcCoA + NADH + CO2
|
|
|
In the TCA cycle, one AcCoA makes what?
|
2 CO2, 3 NADH, FADH2, GTP
|
|
|
What does coenzyme A do?
|
It's a handle for carrying acetate in the TCA cycle.
|
|
|
Write out the TCA cycle:
|
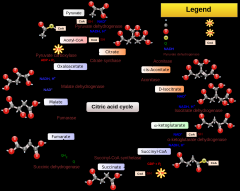
|
|
|
What are the products of the TCA cycle?
|
Primarily reduced electron carriers NADH and FADH2.
|
|
|
What do the reduced electron carriers of the TCA cycle contribute to?
|
They donate electrons to the electron transport chain.
|
|
|
How many complexes in the electron transport chain?
|
4.
|
|
|
Name the 4 complexes of the electron transport chain:
|
1. NADH-CoQ reductase
2. Succinate CoQ reductase 3. CoQH2 Cytochrome C reductase 4. Cytochrome C oxidase |
|
|
As you go through the electron transport chain, the free energy per electron...
|
goes down.
|
|
|
What is the electron carrier between complex I, II, and III of the electron transport chain?
|
Ubiquinone.
|
|
|
What is the free radical version of the ubiquinone called?
|
ubisemiquinone.
|
|
|
A reduced ubiquinone has...
|
an extra H.
|
|
|
What is the electron carrier between complex III and IV of the electron transport chain?
|
Cytochrome C.
|
|
|
What is the chemical process by which cytochrome C carries an electron?
|
Cyt C picks up electrons and becomes reduced and then gives the electrons to complex IV which becomes oxidized.
|
|
|
Why are cytochromes named that?
|
Chrome is due to the heme. They change color when they go from an oxidized to a reduced state. You can track the oxidized and reduced states by monitoring the absorbance of wavelengths.
|
|
|
How do we know the H+ pump works?
|
We can measure the membrane potential and the pH inside the mitochondria.
|
|
|
When O2 is added to a solution containing mitochondria, what happens to the change in H+ concentration.
|
It goes down.
|
|
|
To block the electron transport chain at different steps, you can use...
|
inhibitors.
|
|
|
What blocks complex II?
|
Rotenone, amytal, piericidin A, preogesterone.
|
|
|
What blocks CoQ from getting to complex III?
|
Antimycin
|
|
|
What blocks things from leaving complex IV?
|
Cyanide, azid, carbon monoxide.
|
|
|
Why pump protons in the mitochondria?
|
It is used to make ATP via ATP synthase.
|
|
|
What is the potential change created by the electron transport chain?
|
-0.59 change in pH.
|
|
|
What is the change in phi?
|
The electrical potential as protons are pumped out of the matrix in the electron transport chain.
|
|
|
What 3 things can drive ATP production?
|
1. A pH gradient
2. a membrane potential 3. A combintion |
|
|
How do you describe the proton movie force?
|
Proton Motive Force (pmf) = DY -.059D pH (measured in volts).
|
|
|
What is the total pmf?
|
total pmf = -220 mV (-160 as potential and 1 pH unit)
1 pH unit = ~ 60 mV |
|
|
What creates ATP?
|
ATP synthase
|
|
|
What is the structure of ATP synthase?
|
1. top bulb part: alpha and beta subunits
2. shaft that rotates: gamma shaft 3. the part that makes it rotate: the c subunits |
|
|
What is F1 of the ATP synthase?
|
The bulb that is in the cytoplasm made up of alpha and beta subunits which releases ATP.
|
|
|
What is Fo of the ATP synthase?
|
The section that makes up the c subunits in the membrane.
|
|
|
What do the a and b subunits of the ATP synthase do?
|
They make sure that the Fo and F1 complexes stay together. Also the a subunit has a channel for protons to enter and allow the c subunits to spin.
|
|
|
How is ATP ejected from the ATP synthase?
|
The central shaft (gamma) is a CAM that ejects ATP.
|
|
|
What stimulates the synthesis of uncoupling proteins?
|
Thyroxine.
|
|
|
What is an inhibitor of ATP production?
|
2,4 dinitrophenol.
|
|
|
How does 2,4 DNP inhibit ATP synthesis?
|
It picks up protons and carries them across the membrane leading to a rapid consumption of energy and a decrease in the potential without any ATP made.
|
|
|
What does 2,4 DNP do to the matrix of the mitochondria?
|
It makes it more acidic with the influx of protons.
|
|
|
Mitochondrial damage could start which 3 diseases?
|
1. Alzheimers
2. Parkinson's 3. Lou Gehrig's |
|
|
What 2 mitochondrial mechanisms induce apoptosis?
|
1. Permeability of inner membrane cause mitochondria to swell and burst, known as the MTP pore.
2. Permeability of outer membrane mediated by permeability transition pore (PTP) releases substances from the space between the inner and out membrane |
|
|
What do antiapoptotic proteins prevent in the mitochondria?
|
PTP or the permeability transition pore.
|
|
|
What is reperfusion injury?
|
the tissue damage caused when blood supply returns to the tissue after a period of ischemia or lack of oxygen.
|
|
|
What does reperfusion cause?
|
A massive burst of ROS which causes permanent damage to the heart.
|
|
|
What is the extrinsic pathway to apoptosis?
|
The activation of apoptosis by death receptors on the surface of cells.
|
|
|
What is intrinsic pathway to apoptosis?
|
The Bcl-2 family of proteins release cytochrome C from the mitochondria and they compromise anti-apoptotic and pro-apoptotic proteins.
|
|
|
Which Bcl proteins are proapoptotic?
|
BAX/BID
|
|
|
Which Bcl proteins are antipoptotic?
|
Bcl-2
|
|
|
Where is Bcl-2 bound to?
|
The outer surface of the mitochondria and prevents opening of the PTP pore.
|
|
|
What does BAX/BID do?
|
It's involved in the opening of the PTP pore.
|
|
|
What does the name caspase mean?
|
cysteine aspartyl proteases
|
|
|
What are the 2 primary roles of caspases?
|
1. promote cyt c release with Bcl proteins like BID
2. They are terminal effectors/executioner caspases. |
|
|
What do executioner caspases do?
|
They activate DNases and degrade structural and regulatory proteins.
|
|
|
What 5 mitochondrial proteins are related to cell death?
|
1. Smac/Diablo
2. cyt c 3. pro-caspases 2,3,8,9 4. Endo G 5. AIF |
|
|
What's the pathway for Smac/Diablo?
|
Smac/Diablo + IAP = death
|
|
|
What's the pathway for cyt c from the mitochondria?
|
Cyt c + Apaf-1 + procaspase 9 --> apoptosome --> caspase-9 --> caspase-3 --> caspase-x --> death
|
|
|
What is the pro-caspase pathway?
|
pro-caspase 2,3,8,9 --> caspase 3 --> caspase-x --> death.
Pro-caspases can also lead directly to cell death. |
|
|
Which proteins that go into the mitochrondia promote DNA damage?
|
TR3 and p53
|
|
|
What does smac/diablo stand for?
|
second mitochondria-derived activator of caspases/direct IAP binding protein
|
|
|
What are IAPs?
|
inhibitors of apoptosis. They are part of the balance of factors that prevent apoptosis from getting started. SMAC/DIABLO is a protein that binds to many IAPs and inactivates them.
|
|
|
What does AIF do?
|
(Apoptosis Inducing Factor) causes condensation
of DNA and its degradation. |
|
|
What does endonuclease G do?
|
protein that goes into the nucleus and chops up DNA.
|
|
|
What are nuclear lamins?
|
Intermediate filaments that provide structure for the nucleus.
|
|
|
What are the 3 filaments related to cellular structure?
|
1. microfilaments
2. intermediate filaments 3. microtubules |
|
|
What kind of filament is nuclear lamin?
|
intermediate filament, 10 nm
|
|
|
What are microfilaments made out of?
|
actin, 5nm
|
|
|
What microtubules made out of?
|
alpha, beta tubulin dimers, 25nm
|
|
|
2 kinds of lamins:
|
LaminA
LaminB |
|
|
Describe laminA:
|
Soluble and reinforce the structure made by laminB.
|
|
|
Describe laminB:
|
permanently made hydrophobic and they stay in the nuclear envelope or the ER during mitosis.
|
|
|
3 things that nuclear lamins do during mitosis:
|
1. give shape to the nucleus
2. disassemble when phosphorylated - event in mitosis called the nuclear envelope breakdown at prophase. 3. reassemble when dephosphorylated at end of mitosis (telophase). |
|
|
5 functions of lamina:
|
1. They anchor chromatin and repress trxn.
2. organize and position interphase chromosomes. 3. their defects can cause a loss in heterochromatin 4. defects in DNA repair 5. affects to speed up or delay cell cycle. |
|
|
What disease is due to a defect in the lamins?
|
Hutchinson-Gilford progeria
|
|
|
What controls the direction of transport out of the nucleus?
|
RAN through GTP hydrolysis
|
|
|
Describe the nuclear pores:
|
They're made from ~50 different proteins called nucleoporins.
|
|
|
What is the limit for molecules moving freely out of nucleus?
|
5 KDa or less move freely.
17 KDa slowed. 60 KDa are impermeable. |
|
|
What's the size of a nuclear pore?
|
30 nm
|
|
|
Transport through the pore does not require...
|
energy.
|
|
|
How are large molecules transported into the nucleus?
|
The molecule binds to importin and attaches to FxFG down the pore. Ran-GTP attaches to the carrier and kicks off the molecule.
|
|
|
How is RCC1 related to Ran?
|
RCC1 is a GEF for Ran in the nucleus.
|
|
|
How are large molecules transported out of the cell?
|
Ran-GTP binds to exportin and the cargo and when the complex enters the cytosol Ran-GTP is converted to Ran-GDP and the cargo is released.
|
|
|
What does the Ran GEF do?
|
It converts Ran-GDP to Ran-GTP.
|
|
|
What are karyopherins?
|
Another name for importin, exportin.
|
|
|
Where is Ran-GDP?
|
In the cytosol.
|
|
|
Where is Ran GEF?
|
In the nucleus.
|
|
|
What are nucleolar organizers?
|
Regions of the chromosome containing rDNA which has tandem repeats found in ribosomes.
|
|
|
What does the nucleolus do?
|
It makes ribosomes beginning with the trxn of ribosomal DNA.
|
|
|
How do retroviruses use reverse transcriptase?
|
1. Entry into cell and loss of capsid
2. RNA which makes DNA 3. Reverse transcriptase makes DNA/RNA and then DNA/DNA double helix 4. Integration into the host chromosome creating long terminal repeats. |
|
|
constitutive heterochromatin:
|
Repetitive DNA which is never active.
|
|
|
Facultative chromatin
|
can be active or inactive. Ex. barr body
|
|
|
4 levels of packing of chromosomes:
|
1. double helix
2. beads on a string 3. 30 nm fiber 4. 300 nm loops on scaffolding 5. 700 nm fiber 6. chromosome 1400 nm |
|
|
3 modifications for histone tails:
|
1. acetylation
2. methylation 3. phosphorylation |
|
|
Which histone binds between the nucleosomes?
|
H1
|
|
|
Histone acetylation leads to...
|
gene expression, histone deposition.
|
|
|
Histone methylation leads to...
|
gene silencing.
|
|
|
Histone phosphorylation leads to...
|
mitosis/meiosis.
|
|
|
Where does DNAase attack for the nucleosomes?
|
It attacks in multiples of 200 bp so you get equally distant lines on a gel.
|
|
|
What is the Tunel assay?
|
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) is an established method for detecting DNA fragments.
|
|
|
How does the Tunel assay work?
|
TUNEL labels nicks in the fragmented DNA which floresce.
|
|
|
What is the 30 nm fiber?
|
It's a DNA packing where the nucleosomes go in a zig-zag pattern.
|
|
|
What is a chromosome scaffold?
|
They 30 nm fiber are attached in a loop pattern to the scaffold.
|
|
|
What 3 factors can determine heterochromatin creation?
|
1. chromosome location
2. nuclear localization 3. density of repetitive DNA elements |
|
|
4 examples of heterochromatin:
|
1. centromeres
2. telomeres 3. knobs 4. transposable elements. |
|
|
4 ways heterochromatin influences gene expression:
|
1. change in DNA organization
2. shuts down gene expression close or far away 3. recombination 4. prevents spread of transposable elements |
|
|
What are the 4 steps for modifying histones for heterochromatin?
|
1. Methylation of H3 on Lys 9
2. Methylation recruits HP1. 3. H3 must be deacetylated by Sirtuins 4. Histone methyl transferase and histone deacetylase spread out methylating and deacetylating. |
|
|
How are heterochromatin and epigenetics related?
|
Parts of packaging can be herited by the offspring meaning that though the DNA is not changed, certain genes can be silenced.
|
|
|
How is heterochromatin and sRNAs related?
|
Binding of small RNAs is one way to trigger heterochromatin formation.
|
|
|
What is RNAi?
|
prevent
expression of genes inside the cell. |
|
|
How are small RNAs formed?
|
These small RNAs can be formed by chopping up double stranded RNA inside the cell.
Some of these double stranded RNAs come from transcription of repetitive DNA or viruses |
|
|
3 things needed to be a chromosome:
|
1. origin of replication
2. centromeric DNA 3. Telomeric DNA |
|
|
2 ways to identify chromosomes:
|
1. placement of the centromeres
2. banding pattern |
|
|
3 types of chromosomes:
|
1. metacentric
2. submetacentric 3. acrocentric |
|
|
What is metacentric?
|
The centromeres are in the middle.
|
|
|
What is submetacentric?
|
Centromeres are near the top.
|
|
|
What is acrocentric?
|
Centromeres are on the very top.
|
|
|
What is a karyotype?
|
Chromosomes paired up with their matching partner so they can be identified.
|
|
|
Name 3 chromosome defects:
|
1. Ring chromosome
2. Translocated chromosome 3. Chromosome fragments |
|
|
What did the experiments by George Palade show?
|
It showed the progression of protein movement from ER synthesis to the plasma membrane. He added radioactive amino acids and then washed away the radioactivity. Then used EM to see silver grains where the labeling was.
|
|
|
George Palade found that over time...
|
there was a higher concentration of proteins from the ER to the secretory granules.
|
|
|
What is the MTOC?
|
It is centrosome in the middle of the cell for the microtubules.
|
|
|
3 parts of protein transport from the ER to the golgi:
|
1. coat assembly and disassembly
2. tethering 3. SNARE-SNARE |
|
|
What is coat assembly?
|
COP 1, II and clathrin coat proteins are used at various locations Arf and Sar1 are GTPases that regulate coat assembly
|
|
|
What is tethering?
|
location specific. Tethering concentrates vesicles in different regions, may participate in SNARE assembly, Depends on Rab proteins – a family of small G proteins.
|
|
|
What does SNARE-SNARE do?
|
interactions and membrane fusion
|
|
|
6 steps of the vesicle life cycle:
|
1. Cargo interacts with coat
2. Coat assembles 3. Vesicle scission 4. Vesicle transport 5. Tethering / Uncoating 6. Fusion |
|
|
What proteins regulate coat assembly?
|
Sar1 and ARF in the ER-golgi vesicle transport.
|
|
|
Which proteins make the coat?
|
COPI and COPII.
|
|
|
What protein makes the coat leaving the ER?
|
COP II
|
|
|
How does COPII make the coat?
|
The donor membrane from the ER has a GEF and Sar1 binds to GTP and sticks onto membrane and then COPII coats the vesicle.
|
|
|
What are Rab proteins and tethers?
|
They're proteins on the surface of the golgi that capture vesicles
|
|
|
What does the COP I coat do?
|
It takes it back from the golgi to the ER.
|
|
|
What is the pH of the ER?
|
Neutral, around 7.
|
|
|
What is the pH of the Golgi stacks?
|
Acidic
|
|
|
What is the pH of the vesicular tubular cluster?
|
Weakly acidic.
|
|
|
What are the 2 parts of the golgi?
|
Cis and trans.
|
|
|
Which part of the golgi is closest to the ER?
|
The cis part.
|
|
|
What does KDEL mean?
|
It's the signal sequence that specifies the retention of the ER through a retrieval mechanism, ie by interacting with COPI.
|
|
|
How does the trans side of the golgi relate to the nucleus?
|
It faces away from the nucleus.
|
|
|
What are the 5 levels going through the golgi?
|
1. Phosphate sugars
2. Remove Man 3. Remove Man, add GlcNAc 4. Add Gal 5. Add NANA |
|
|
What are the 2 models for golgi movement?
|
1. Stable compartment
2. Cisternal maturation |
|
|
What is the stable compartment model?
|
The golgi is made up of stable compartments in which large and small cargo are transported to each compartment.
|
|
|
What is the cisternal maturation model?
|
The golgi matures from the cis to the trans via golgi proteins.
|
|
|
What is the prove that one model for golgi is right?
|
If the stable compartment model is right, there should only be cargo in vesicles. If the cisternal maturation is right, there should only be golgi enzymes in vesicles. Some researchers have detected cargo in COPI.
|
|
|
Which proteins can leave the golgi?
|
Proteins with long transmembrane stretches.
|
|
|
How is clathrin related to transport from the golgi?
|
Clathrin-coated vesicles deliver to endosomal/ysosomal/vaculoar system.
|
|
|
What protein recruits clathrin?
|
Adaptins
|
|
|
How are adaptins related to transport specificity?
|
Different adaptins are associated with different destinations from the golgi.
|
|
|
What is the structure of clathrin?
|
It's a triskelion with 3 heavy chains and 3 light chains which assemble in hexagons.
|
|
|
What do tubulating proteins do?
|
They help make vesicles.
|
|
|
How do tubulating proteins help make clathrin vesicle?
|
Proteins containing BAR domains cause the membrane to bend. Then dynamin makes a collar around the tubes and eventually the vesicle is released.
|
|
|
What proteins regulate making very large vesicles?
|
Actin coats and rings generate large secretory vesicles.
|
|
|
How does LDL work?
|
LDL connects to the membrane by LDL receptors which creates a vesicle and is then uncoated. It fuses to an endosome and the cholesterol is put into a lysosome with hydrolytic enzymes. The receptors are recycled back to the membrane.
|
|
|
What does golgi add to the lysosomal hydrolase precursor from the ER?
|
mannose-6-phosphate.
|
|
|
Why does golgi use mannose-6-phosphate?
|
The cargo will bind to an M6P receptor on the trans side which helps create vesicles.
|
|
|
Where does the M6P vesicle go after the golgi?
|
To the late endosome where the phosphate dissociates and creates a mature lysosomal hydrolase.
|
|
|
What is I cell disease?
|
Defective enzyme for making M6P. It's a lysosome storage disease where lysosomal enzymes are secreted out of the cell and don't get to lysosomes.
|
|
|
Hurler's Syndrome:
|
A defective M6P receptor.
|
|
|
5 functions of the ER:
|
1. lipid synthesis
2. Release of fatty acids to be oxidized 3. Detoxification 4. Calcium transport, storage, and release 5. Cotranslational insertion and modification of proteins |
|
|
What are 5 ways the ER can modify proteins?
|
1. cotranslational insertion
2. glycosylation 3. proteolysis 4. disulfide bonds and folding reactions 5. transport to golgi |
|
|
5 steps of protein-ribosome mRNA complexes to the ER:
|
1. translation of protein exposing N terminal signal sequence
2. SRP binds to N terminal signal 3. Trsln stops 4. SRP takes peptide-ribosome-mRNA complex to ER and it binds to SRP and is transferred to translocon. 5. Trsln starts again in translocon and protein is fed through translocon into ER. |
|
|
What is SRP
|
It's a riboprotein that binds to N terminal signal sequences and pauses trsln
|
|
|
5 steps of SRP:
|
1. SRP binds to signal sequence
2. Binding pauses trsln 3. SRP-bound ribosome attaches to SRP receptor in ER membrane. 4. Trsln continues and translocation begins 5. SRP and SRP receptor and displaced and recycled. |
|
|
What attaches to the protein translocator of the ER?
|
The ribosome and there's a channel in the large ribosomal subunit.
|
|
|
2 facts about the ER translocon pore:
|
1. A new peptide can be chemically cross-linked to proteins on the wall of the translocon.
2. The new peptide is in an aqueous environment. |
|
|
If the N-terminal of the peptide is positive, where does it go?
|
Outside of the ER.
|
|
|
If the N-terminal of the peptide is negative, where does it go?
|
Into the ER.
|
|
|
What does a positive part of the N-terminal signal do?
|
It stops the protein to be on the outer part of the ER membrane.
|
|
|
What does the stop sequence of a translocated protein do?
|
It stops the loop of the peptide in the membrane.
|
|
|
If the N terminal is - +, which part of the peptide will be in the cytosol?
|
The C terminal. The N terminal will be in the ER lumen.
|
|
|
4 kinds of membrane proteins with the N terminal inside:
|
1. glycophorin
2. LDL receptor 3. Influenza HA protein 4. insulin receptor 5. cyt p450 |
|
|
3 kinds of proteins with the N terminal on the outside:
|
1. transferrin receptor
2. sucrase-isomaltase precursor 3. influenza HN protein 4. cyt b5 |
|
|
What kind of membrane protein is GLUT1?
|
It's a transmembrane protein that crosses 12 times and has N and C terminal on the outside.
|
|
|
What are O-linked proteins?
|
They are proteins with 1-4 sugars that are added on one by one.
|
|
|
What is N-linked protein?
|
Sugars are added onto the dolichol which are then transferred as a chain to N.
|
|
|
3 things sugars are used for for proteins:
|
1. protection from proteases
2. Stability 3. recognition |
|
|
What are N-linked sugars added onto before the protein?
|
Dolichol.
|
|
|
What must happen before dolichol transfers the sugars?
|
It must flip into the ER lumen and then transfer it to the protein.
|
|
|
What is "trimming"?
|
Removing glucose.
|
|
|
How are disulfide bonds formed in the lumen of the ER?
|
Disulfide isomerase which makes sure the correct disulfide bonds are made.
|
|
|
What are GPI proteins?
|
Proteins that bind inside the lumen to a GPI anchor with glycosylphosphatidylinositol.
|
|
|
4 things microtubules do:
|
1. organize in the cytoplasm
2. Highways for traffic 3. Form the heart of spindle machinery for mitosis 4. movement of flagella and cilia |
|
|
What are the components of tubulin?
|
Heterodimer made of alpha, beta. A special form called gamma tubulin is at the base of the microtubules in the centrosome region.
|
|
|
What does gamma tubulin form?
|
It makes rings to start microtubules.
|
|
|
What do the tubulin subunits do?
|
They bind GTP but only beta hydrolyzes GTP.
|
|
|
What are the linear chains of alpha beta dimers called?
|
protofilaments.
|
|
|
How is the growing microtubule unstable?
|
As tubulin dimers add to the microtubule, the bound GTP is hydrolyzed and the microtubule becomes unstable.
|
|
|
2 depolymerizing drugs for tubulin:
|
1. colchicine
2. nocodazole |
|
|
Drug for stabilizing tubulin
|
taxol
|
|
|
microtubules are composed of how many protofilaments?
|
13.
|
|
|
What are the different states of microtubule formation according to GTP?
|
GTP-GTP high formation
GTP-GDP medium formation GDP-GDP no formation |
|
|
Nucleation vs. elongation of microtubules.
|
Nucleation is Slow, Elongation is Fast
|
|
|
Where is the gamma tubulin?
|
At the minus ends.
|
|
|
3 steps of microtubule assembly:
|
1. protofilament assembly
2. sheet assembly 3. microtubule elongation |
|
|
+ end vs. - end:
|
Addition and loss is faster at the + end.
|
|
|
Where is there more likely to be GDP on the microtubule?
|
At the - end.
|
|
|
What is CC+?
|
No net growth at the plus end of the microtubule.
|
|
|
What is CC-?
|
No net growth at the minus end of the - end.
|
|
|
What is CCo?
|
No net growth or shrink for whole microtubule.
|
|
|
What is the MTOC made out of?
|
Centrioles.
|
|
|
What caps microtubules at the - end?
|
Probably ninein.
|
|
|
Why does the microtubule break down at the + end?
|
When the GTP cap is hydrolized to GDP, the microtubule breaks down catastrophically.
|
|
|
What does the kinetochore bind?
|
TIPS
|
|
|
How are MAPS related to microtubules?
|
They form crosslinks between microtubules.
|
|
|
What are motor MAPS?
|
Motors that use ATP for movement: Dynein and Kinesin.
|
|
|
Which was does dynein go?
|
Toward the cell body.
|
|
|
3 parts of kinesin:
|
1. globular heads
2. light chain which binds vesicle 3. coiled alpha helix body |
|
|
How does ATP help kinesin?
|
It helps it bind to the microtubule.
|
|
|
How many heads does dynein have?
|
3.
|
|
|
Structure of flagella:
|
1. 9 doublet microtubules
2. 2 singlet microtubules. |
|
|
How do flagella move?
|
Microtubules move up and down and get closer and farther apart based on proteolyzed crosslinks.
|

