![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
127 Cards in this Set
- Front
- Back
|
define "homeostasis"
|
the condition in which the body's internal environment remains relatively constant within certain physiological limits
|
|
|
characteristics of a "steady state"
|
1. open system to matter and heat
2. dynamic 3. living |
|
|
characteristics of a "chemical equilibrium"
|
1. closed system to matter
2. static -- unchanging 3. non living |
|
|
the 6 components of a generic feedback system
|
1. sensor
2. circuit 3. effector 4. response 5. feedback 6. shut-off |
|
|
function of a sensor
|
detect change
|
|
|
function of a circuit
|
carries the signal
|
|
|
function of an effector
|
produces an effect; it does something
|
|
|
function of response
|
the output of the effector
|
|
|
function of feedback
|
it is the response detected by the sensor
|
|
|
function of shut-off
|
the sensor reacting to normalization
|
|
|
list the components involved in the body's response to cold/warm temperatures
|
1. receptors - in skin (peripheral) and in brain (central)
2. circuit - nerves 3. effectors - glands or muscles (in cold: shivering, goosebumps, peripheral vasoconstriction, increased thyroid hormone; in warm: sweat glands, peripheral vasodilation, decreased thyroid hormone) 4. response - the secretion by a gland or the contraction/relaxation of a muscle 5. feedback - peripheral and central receptors detect normal temp 6. shut off - the receptors in the skin stop sending the signal bc body temp is back to normal |
|
|
"negative feedback" vs "positive feedback"
|
in negative feedback, the response (to the change in the system) REVERSES the stimulus; it is STABILIZING and is the most common type in living beings to keep us in homeostasis! (ie. blood pressure, blood sugar, etc.)
in positive feedback, the response ENHANCES the stimulus (same direction), it is DESTABILIZING (ie. blood clotting and childbirth) |
|
|
homeostasis is the heart of all organ systems EXCEPT ___________________________.
|
the reproductive system
|
|
|
5 basic components of a eukaryotic cell
|
1. cell membrane (plasma membrane, plasmalemma)
2. cytoplasm 3. inclusions 4. organelles 5. cell wall |
|
|
the structure of the cytoplasm
|
1. the cytosol is the liquid part
2. the cytoskeleton is the non liquid part; consists of microtubules, microfilaments, and intermediate filaments. it maintains the cell's integrity (structure) and is involved in movement, both inside and outside the cell. |
|
|
define the term "inclusions"
|
storage products made by the cell and stored in the cytoplasm (ie. glycogen and melanin)
|
|
|
what is the function of the nucleus
|
contains the genetic information of the cell --- chromosomes, genes, DNA
|
|
|
what is the function of ribosomes
|
they are where proteins are made in the cell
|
|
|
what is the function of the endoplasmic reticulum
|
intracellular transport
|
|
|
what is the function of mitochondria
|
stores energy from nutrients as ATP
|
|
|
what is the function of the Golgi complex
|
modifies protein structure as proteins move through it
|
|
|
what is the function of lysosomes
|
intracellular breakdown/digestion
|
|
|
what is the function of peroxisomes
|
detoxify by oxidation
|
|
|
what is the function of flagella
|
movement (motility), involved in swimming [ie. sperm cells]
|
|
|
what is the function of cilia
|
movement (motility) and they also sweep things past the cell [ie. in the throat]
|
|
|
what is the function of centrioles
|
guide chromosomes during cell division
|
|
|
what is the function of chloroplasts
|
they're involved in photosynthesis
|
|
|
what is the difference between cilia and flagella
|
flagella are few and long; cilia are short and there are many
|
|
|
list the type of organisms in which a cell wall is found
|
plants, fungi, bacteria and algae --- NOT in humans/animals or protozoa
|
|
|
what is the difference between smooth ER and rough ER?
|
rough ER has ribosomes attached to it, giving it a "rough" appearance; smooth ER has no ribosomes attached
|
|
|
what is the difference between chromosomes, genes, and DNA?
|
a gene resides on a chromosome and codes for a specific protein, and multiple chromosomes make up a strand of DNA, which is the molecular code that codes for all proteins which make up life.
|
|
|
define matter
|
anything that takes up space and has mass
|
|
|
define element
|
the simplest form of matter that cannot be broken into a simpler substance by a chemical reaction
|
|
|
define atom
|
basic units of matter that are made up of protons, neutrons, and electrons
|
|
|
define atomic number
|
number of protons in the nucleus
|
|
|
define atomic mass
|
the total mass of the atom (p+n+e); it is the average of all isotopes
|
|
|
define mass number
|
number of particles in the nucleus (protons+neutrons)
|
|
|
define isotope
|
atoms of the same element with different numbers of neutrons; some are radioactive
|
|
|
list 3 uses for radioactive isotopes
|
treat cancer, detect cancer, date fossils
|
|
|
list the major elements of the body
|
carbon, hydrogen, oxygen, nitrogen, sulfur, phosphorus
|
|
|
list the 3 principle subatomic particles, along with the charge each carries
|
protons - positive
neutrons - neutral electrons - negative |
|
|
list the parts of atoms involved in chemical reactions
|
electrons, specifically the ones in the outer (valence) shell
|
|
|
state the number of electrons permitted in the first three energy levels
|
level K: 2
level L: 8 level M: 18 for a total of 28 |
|
|
what are the three major types of chemical bonds?
|
Ionic, Covalent, Hydrogen
|
|
|
list the three major types of chemical bonds in order of strength
|
weakest: hydrogen
intermediate: ionic strongest: covalent |
|
|
define ionic bonds
|
one element gives up electron(s) to another element; this produces ions (cations + and anions -); non-directional; intermediate strength [[ie. NaCl gives us Na+ and Cl-]]
|
|
|
define covalent bonds
|
valence electrons are shared between elements; directional bond (atom only attracts one other atom); strongest bond; most important bond from a biological standpoint [[ie. CH4 (methane)]]
|
|
|
define hydrogen bonds
|
this bond occurs between a Hydrogen in one polar covalent bond and another atom (usually an Oxygen or Nitrogen in another polar covalent bond); directional bond; weakest [[ie. lots of water molecules -- the hydrogens from one compound is also bonded with another oxygen from a different compound]]
|
|
|
contrast the two types of covalent bonds
|
polar covalent: 1 atom has greater attraction (electronegativity) for electrons than the other when they share (one atom has more of a positive charge) [[ie. one water molecule]]
nonpolar covalent: the atoms equally share the electrons [[ie. H+ and H+]] |
|
|
define the term "electronegativity"
|
the tendency of an atom to attract electrons in the formation of a bond
|
|
|
contrast "molecule" and "compound"
|
molecule: 2 or more atoms bound together
compound: 2 or more DIFFERENT atoms bound together |
|
|
the octet rule
|
beginning with the second energy level (because the first one can only hold 2) ---> 8 electrons = stable
|
|
|
valence
|
the number of extra or missing electrons in the outermost energy level
|
|
|
what are inert elements?
|
noble gases; they're unreactive because they have 8 electrons in their valence shell, thus they have no need to bond with other elements
|
|
|
why is water the "universal solvent?"
|
water is found everywhere ; it's an aqueous solution and its hydrogen bonding gives water unique properties
|
|
|
give 5 reasons why water is the universal solvent:
|
1. cohesion - water molecules are attracted to each other
2. adhesion - water molecules also stick to other molecules 3. high heat capacity - water needs lots of heat to raise its temperature 4. high heat of vaporization 5. water expands when it freezes 6. water ionizes |
|
|
what is the difference between "organic" and "inorganic" compounds?
|
organic compounds contain both carbon and hydrogen; inorganic compounds do not
|
|
|
what does it mean if something is hydrophilic?
|
"water loving"
|
|
|
what does it mean if something is hydrophobic?
|
"water-fearing"
|
|
|
what is a free radical?
|
organic compounds in which a carbon atom has one unpaired electron -- "open bond." they're extremely reactive.
-yl ie. CH3CH2• |
|
|
what is a hydrocarbon?
|
hydrocarbons contain only hydrogen and carbon; divided into three groups (based on the types of bonds joining the carbon atoms): alkanes, alkenes, alkynes
|
|
|
what types of bonds do alkanes, alkenes, and alkynes have?
|
alkanes - ONLY single bonds between carbon atoms
alkenes - at least one double bond between carbon atoms alkynes - at least one triple bond between carbon atoms |
|
|
what are alcohols?
|
they contain hydroxyl groups (OH). their general formula is ROH, where "R" represents a radical
-ol the carbon bonded to the hydroxyl group is given the lowest # possible |
|
|
what is the difference between terminal alcohols and nonterminal alcohols?
|
Terminal alcohols have the hydroxyl group on the end (ie. 1-propanol)
Nonterminal alcohols have the hydroxyl group on the interior (ie. 2-propanol) |
|
|
what is an ether?
|
Ethers are formed by dehydration or condensation reaction between 2 alcohol compounds, in which water is removed by combining the hydroxyl (OH) group of one alcohol with the hydrogen atom from the hydroxyl group of the other.
The general formula is ROR They are named by naming the two radicals (in alphabetical order), then adding the word "ether." |
|
|
what are aldehydes?
|
Aldehydes are produced by oxidation of terminal alcohols
look for CHO (the aldehyde or formyl group) with the carbon double bonded to the oxygen -al the aldehyde group must be included in finding the carbon backbone |
|
|
what are ketones?
|
Ketones are formed when non-terminal alcohols are oxidized by removal of hydrogen atoms (a double bond forms to the oxygen atom), and the resulting CO group is called the ketone group
the ketone group must be included in finding the carbon backbone the carbon # is indicated, followed by the root of the name and the ending "one" |
|
|
what are organic (carboxylic) acids?
|
carboxylic acids form when aldehydes are further oxidized by the addition of oxygen. this results in the formation of the COOH group called a carboxyl group.
the carbon that is part of the carboxyl group must be included in the carbon backbone -oic acid |
|
|
how many carbons does the prefix "meth" have?
|
1
|
|
|
how many carbons does the prefix "eth" have?
|
2
|
|
|
how many carbons does the prefix "prop" have?
|
3
|
|
|
how many carbons does the prefix "but" have?
|
4
|
|
|
how many carbons does the prefix "pent" have?
|
5
|
|
|
how many carbons does the prefix "hex" have?
|
6
|
|
|
how many carbons does the prefix "hept" have?
|
7
|
|
|
how many carbons does the prefix "oct" have?
|
8
|
|
|
how many carbons does the prefix "non" have?
|
9
|
|
|
how many carbons does the prefix "dec" have?
|
10
|
|
|
what is the general formula for radicals?
|
R
Carbon + hydrogen where there's one unpaired electron |
|
|
what is the general formula for hydrocarbons?
|
Carbon & Hydrogen ONLY
|
|
|
what is the general formula for alcohols?
|
ROH
|
|
|
what is the general formula for ethers?
|
ROR
|
|
|
what is the general formula for aldehydes?
|
RCHO
|
|
|
what is the general formula for ketones?
|
RR'CO
|
|
|
what is the general formula for organic (carboxylic) acids?
|
RCOOH
|
|
|
what is the general formula for alkanes?
|
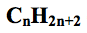
|
|
|
what is the general formula for alkenes?
|
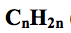
|
|
|
what is the general formula for alkynes?
|
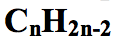
|
|
|
how are ethers formed?
|
Ethers are formed by a dehydration/condensation reaction between two alcohols; water is removed, leaving ROR
|
|
|
how are aldehydes formed?
|
oxidation of terminal alcohols
RCHO |
|
|
how are ketones formed?
|
oxidation by removal of hydrogen atoms in non-terminal alcohols
RCO |
|
|
how are organic (carboxylic) acids formed?
|
when aldehydes are further oxidized by the addition of oxygen
COOH |
|
|
name some simple examples of organic molecules
|
butane, propanoic acid, propanal, ethanol, propanone
|
|
|
what are the major categories of carbohydrates?
|
monosaccharides, disaccharides, polysaccharides
|
|
|
give 3 examples for each major category of carbohydrates
|
monosaccharides: glucose, fructose, galactose
all are hexoses (have 6 carbons) C6H12O6 disaccharides: maltose, sucrose, lactose C12H22O11 polysaccharides: glycogen, starch, cellulose (& chitin) |
|
|
contrast the terms "hydrolysis" and "dehydration synthesis"
|
hydrolysis - adding water
dehydration (condensation) - removal of water |
|
|
define the term "isomer"
|
chemical compounds with the same formula but different structures
|
|
|
what is the component monosaccharides for maltose?
|
glucose + glucose
|
|
|
what is the component monosaccharides for sucrose?
|
glucose + fructose
|
|
|
what is the component monosaccharides for lactose?
|
glucose + galactose
|
|
|
what are the differences between glycogen, starch, and cellulose?
|
glycogen is how animals store glucose (in liver and muscle cells)
starch is how plants store glucose cellulose gives plants STRUCTURE, not storage! |
|
|
list the 8 types of lipids
|
1. triglycerides
2. phospholipids 3. waxes 4. sterols 5. lipoproteins 6. eicosanoids 7. carotenoids 8. fat-soluble vitamins (A, D, E, K) |
|
|
list the building blocks of triglycerides
|
glycerol + 3 fatty acids
|
|
|
define the term "saturation"
|
only single bonds, thus the carbons are "saturated" with hydrogen
|
|
|
what are the 3 functions of triglycerides?
|
energy, insulation, padding
|
|
|
what are the 7 functions of proteins?
|
1. structural
2. enzymes 3. regulatory 4. contractile 5. immunological 6. transport 7. energy |
|
|
what are the building blocks of proteins?
|
amino acids
|
|
|
what elements do proteins contain?
|
C, H, O, N, S
|
|
|
what is the general structure of an amino acid?
|
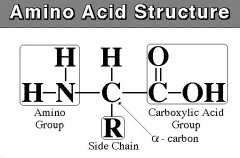
|
|
|
what is the name of the bond joining amino acids together?
|
peptide bond; it is the carbon of the carboxyl group on one amino acid + the nitrogen from the amino group on another amino acid
|
|
|
what are the four levels of protein organization?
|
1. primary
2. secondary 3. tertiary 4. quaternary |
|
|
define the four levels of protein organization
|
1. primary: the sequence (order) the amino acids are in
2. secondary: repetitions in the structure (due to H bonds between nearby amino acids) 3. tertiary: the overall shape of the protein in 3D space [ due to interactions between distant R groups] 4. quaternary: the polypeptide arrangement [in proteins that have more than one polypeptide] |
|
|
what is denaturation?
|
disruption of H bonds (via heat, radiation, chemicals, etc.); alters the shape and function. ie. an egg
|
|
|
define the term "enzyme"
|
globular proteins [all coiled up -- tertiary structure!] that act as biological catalysts
|
|
|
list the components of enzymes
|
apoenzyme: the protein
cofactor: an inorganic compound coenzyme: an organic compound holoenzyme: the complete, functional enzyme apoenzyme + cofactor or coenzyme = holoenzyme |
|
|
list 5 characteristics of enzymes
|
1. unchanged
2. specific 3. catalyze in both directions [bring together & break apart] 4. lower activation energy 5. can't catalyze impossible reactions [ones that wouldn't happen anyway] |
|
|
list the name of the site to which a substrate binds on an enzyme
|
active site
|
|
|
what are the 2 mechanisms of enzyme action?
|
1. lock and key hypothesis
2. induced fit hypothesis |
|
|
describe the lock and key hypothesis
|
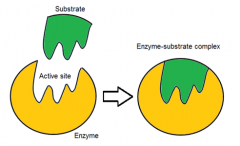
specific substrates (key) fit specific active sites (lock) perfectly like a lock and a key
|
|
|
describe the induced fit hypothesis
|
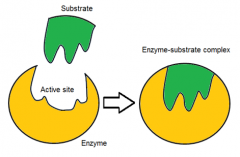
the enzyme's active site changes shape to "better fit" the substrate
|
|
|
list 5 factors affecting the rate of enzyme-catalyzed reactions
|
1. temperature
2. pH 3. concentration of reactant molecules 4. inhibitors 5. thermodynamics |
|
|
define the term "exergonic"
|
energy releasing
the products of the reaction will have LESS energy than the reactants [because energy has been released] ie. hand warmers, striking a match |
|
|
define the term "endergonic"
|
energy gaining
products contain MORE energy than the reactants [because energy is gained] ie. instant cold pack |
|
|
what do all chemical reactions require to start the reaction?
|
activation energy
|
|
|
reactions happen faster if activation energy is lowered by ____________________.
|
catalysts
|
|
|
what do enzymes do?
|
1. increase collisions
2. lower activation energy 3. orients reactions |
|
|
contrast the two types of inhibitors
|
1. competitive: interferes with active site of enzyme so substrate cannot bind; competes with the substrate so the reaction doesn't happen
2. noncompetitive: changes shape of the enzyme so it cannot bind to the substrate; binds to enzyme outside of the active site |

