![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
59 Cards in this Set
- Front
- Back
|
Classification of Organism:
|
Organisms can be classified based on how they obtain energy:
•Autotrophs –Able to produce their own organic molecules through photosynthesis •Heterotrophs –Live on organic compounds produced by other organisms •All organisms use cellular respiration to extract energy from organic molecules |
|
|
Cellular respiration:
|
is a series of reactions
|
|
|
Oxidations:
|
–loss of electrons
|
|
|
Dehydrogenations:
|
lost electrons are accompanied by protons
–A hydrogen atom is lost (1 electron, 1 proton) |
|
|
During redox reactions
|
electrons carry energy from one molecule to another
•Nicotinamide adenosine dinucleotide (NAD+) –An electron carrier –NAD+accepts 2 electrons and 1 proton to become NADH –Reaction is reversible |
|
|
Redox Reaction:
|
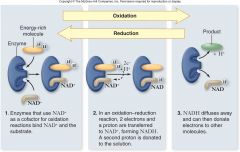
|
|
|
Cellular Energy:
|
harvest
–Dozens or redox reactions take place –Number of electron acceptors including NAD+ •In the end, high-energy electrons from initial chemical bonds have lost much of their energy •Transferred to a final electron acceptor |
|
|
Respiration:
|
Aerobic respiration
–Final electron receptor is oxygen (O2) •Anaerobic respiration –Final electron acceptor is an inorganic molecule (not O2) •Fermentation –Final electron acceptor is an organic molecule |
|
|
Respiration:
|
C6H12O6+ 6O26CO2+ 6H2OG = -686kcal/mol of glucose G can be even higher than this in a cell
•This large amount of energy must be released in small steps rather than all at once. |
|
|
Respiration:
|
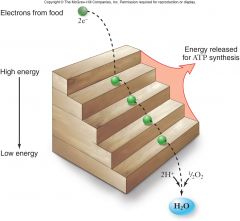
|
|
|
Electron carriers:
|
Many types of carriers used
–Soluble, membrane-bound, move within membrane •All carriers can be easily oxidized and reduced •Some carry just electrons, some electrons and protons •NAD+ acquires 2 electrons and a proton to become NADH |
|
|
Electron Carriers:
Reduction & Oxidation |
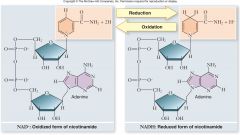
|
|
|
ATP:
|
ATP( plays a central role in metabolism)
•Cells use ATP to drive endergonicreactions –ΔG = -7.3 kcal/mol •2 mechanisms for synthesis 1.Substrate-level phosphorylation •Transfer phosphate group directly to ADP •During glycolysis 2.Oxidative phosphorylation •ATP synthaseuses energy from a proton gradient |
|
|
ATP:
|

|
|
|
Respiration(Oxidation of Glucose):
|
The complete oxidation of glucose proceeds in stages:
1. Glycolysis 2. Pyruvate oxidation 3. Krebs cycle 4. Electron transport chain & chemiosmosis |
|
|
Respiration:
|
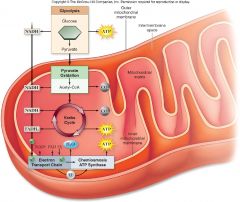
|
|
|
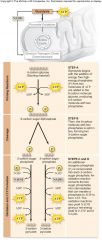
Glycolysis:
|
Converts 1 glucose (6 carbons) to 2 pyruvate (3 carbons)
•10-step biochemical pathway •Occurs in the cytoplasm •Netproduction of 2 ATP molecules by substrate-level phosphorylation •2 NADH produced by the reduction of NAD+ |
|
|
Glycolysis:
|
|
|
|
NADH must be recycled:
|
For glycolysis to continue, NADH must be recycled to NAD+by either:
1.Aerobic respiration –Oxygen is available as the final electron acceptor –Produces significant amount of ATP 2.Fermentation –Occurs when oxygen is not available –Organic molecule is the final electron acceptor |
|
|
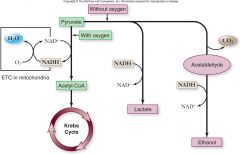
Fate of pyruvate:
|
Depends on oxygen availability.
–When oxygen is present, pyruvate is oxidized to acetyl-CoA which enters the Krebs cycle •Aerobic respiration –Without oxygen, pyruvate is reduced in order to oxidize NADH back to NAD+ •Fermentation |
|
|
Pyruvate:
|
|
|
|
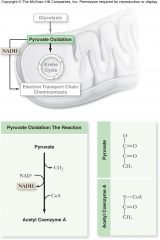
PyruvateOxidation:
|
In the presence of oxygen, pyruvate is oxidized
–Occurs in the mitochondria in eukaryotes •multienzyme complex called pyruvate dehydrogenase catalyzes the reaction –Occurs at the plasma membrane in prokaryotes |
|
|
Pyruvate dehydrogenase:
|
?
|
|
|
Products of pyruvate oxidation:
|
For each 3 carbon pyruvate molecule:
–1 CO2 •Decarboxylation by pyruvate dehydrogenase –1 NADH –1 acetyl-CoA which consists of 2 carbons from pyruvate attached to coenzyme A •Acetyl-CoA proceeds to the Krebs cycle |
|
|
Pyruvate Oxidation:
|
|
|
|
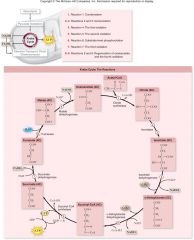
Krebs Cycle:
|
•Oxidizes the acetyl group from pyruvate
•Occurs in the matrix of the mitochondria •Biochemical pathway of 9 steps in three segments 1.Acetyl-CoA + oxaloacetate → citrate 2.Citrate rearrangement and decarboxylation 3.Regeneration of oxaloacetate |
|
|
Krebs Cycle:
|
For each Acetyl-CoA entering:
–Release 2 molecules of CO2 –Reduce 3 NAD+to 3 NADH –Reduce 1 FAD (electron carrier) to FADH2 –Produce 1 ATP –Regenerate oxaloacetate |
|
|
Krebs Cycle:
|
|
|
|
At this point:
|
•Glucose has been oxidized to:
–6 CO2 –4 ATP –10 NADH –2 FADH2 •Electron transfer has released 53kcal/mol of energy by gradual energy extraction •Energy will be put to use to manufacture ATP |
|
|
Electron Transport Chain:
|
ETC is a series of membrane-bound electron carriers
•Embedded in the inner mitochondrial membrane •Electrons from NADH and FADH2are transferred to complexes of the ETC •Each complex –A proton pump creating proton gradient –Transfers electrons to next carrier |
|
|
Electron Transport Chain
|
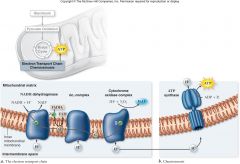
|
|
|
Chemiosmosis:
|
Accumulation of protons in the intermembrane space drives protons into the matrix via diffusion
•Membrane relatively impermeable to ions •Most protons can only reenter matrix through ATP synthase –Uses energy of gradient to make ATP from ADP + Pi |
|
|
Chemiosis
|
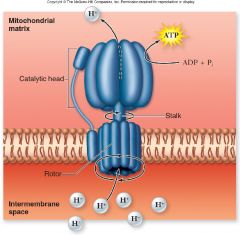
|
|
|
Energy Yield of Respiration:
|
•Theoretical energy yield
–38 ATP per glucose for bacteria –36 ATP per glucose for eukaryotes •Actual energy yield –30 ATP per glucose for eukaryotes –Reduced yield is due to •“Leaky” inner membrane •Use of the proton gradient |
|
|
Energy Yield of Respiration
|
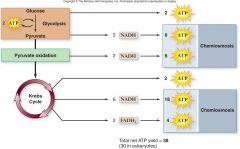
|
|
|
Regulation of Respiration:
|
•Example of feedback inhibition
•2 key control points 1.In glycolysis •Phosphofructokinase is allosterically inhibited by ATP and/or citrate 2.In pyruvate oxidation •Pyruvate dehydrogenase inhibited by high levels of NADH •Citrate synthetase inhibited by high levels of ATP |
|
|
Respiration
|
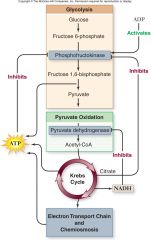
|
|
|
Oxidation Without O2:
|
1.Anaerobic respiration
–Use of inorganic molecules (other than O2) as final electron acceptor –Many prokaryotes use sulfur, nitrate, carbon dioxide or even inorganic metals 2.Fermentation –Use of organic molecules as final electron acceptor |
|
|
Anaerobic respiration:
|
•Methanogens
–CO2is reduced to CH4(methane) –Found in diverse organisms including cows •Sulfur bacteria –Inorganic sulphate (SO4) is reduced to hydrogen sulfide (H2S) –Early sulfate reducers set the stage for evolution of photosynthesis |
|
|
Fermentation:
|
Reduces organic molecules in order to regenerate NAD+
1.Ethanol fermentation occurs in yeast –CO2, ethanol, and NAD+are produced 2.Lactic acid fermentation –Occurs in animal cells (especially muscles) –Electrons are transferred from NADH to pyruvate to produce lactic acid |
|
|
Fermentation
|
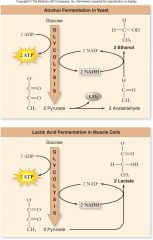
|
|
|
Catabolism of Protein:
|
•Amino acids undergo deamination to remove the amino group
•Remainder of the amino acid is converted to a molecule that enters glycolysis or the Krebs cycle –Alanine is converted to pyruvate –Aspartate is converted to oxaloacetate |
|
|
Catabolism of Protein
|
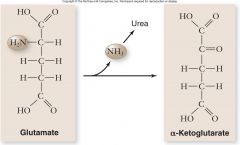
|
|
|
Catabolism of Fat:
|
Fats are broken down to fatty acids and glycerol
–Fatty acids are converted to acetyl groups by beta-oxidation –Oxygen-dependent process •The respiration of a 6-carbon fatty acid yields 20% more energy than 6-carbon glucose. |
|
|
Catabolism
|
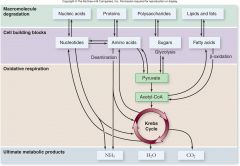
|
|
|
Evolution of Metabolism:
|
Hypothetical timeline:
1.Ability to store chemical energy in ATP 2.Evolution of glycolysis •Pathway found in all living organisms 3.Anaerobic photosynthesis (using H2S) 4.Use of H2O in photosynthesis (not H2S) •Begins permanent change in Earth’s atmosphere 5.Evolution of nitrogen fixation 6.Aerobic respiration evolved most recently |
|
|
Photosynthesis Overview:
|
Energy for all life on Earth ultimately comes from photosynthesis
6CO2+ 12H2O C6H12O6+ 6H2O + 6O2 •Oxygenic photosynthesis is carried out by –Cyanobacteria –7 groups of algae –All land plants –chloroplasts |
|
|
Chloroplast
|
Thylakoid membrane –internal membrane
–Contains chlorophyll and other photosynthetic pigments –Pigments clustered into photosystems •Grana –stacks of flattened sacs of thylakoid membrane •Stroma lamella –connect grana •Stroma –semiliquid surrounding thylakoid membranes |
|
|
Stages:
|
Light-dependent reactions
–Require light 1.Capture energy from sunlight 2.Make ATP and reduce NADP+to NADPH •Carbon fixation reactions or light-independent reactions –Does not require light 3.Use ATP and NADPH to synthesize organic molecules from CO2 |
|
|
Discovery of Photosynthesis:
|
Jan Baptista van Helmont (1580–1644)
–Demonstrated that the substance of the plant was not produced only from the soil •Joseph Priestly (1733–1804) –Living vegetation adds something to the air •Jan Ingen-Housz (1730–1799) –Proposed plants carry out a process that uses sunlight to split carbon dioxide into carbon and oxygen (O2gas) |
|
|
Discovery of Photosynthesis:
|
F.F. Blackman (1866–1947)
–Came to the startling conclusion that photosynthesis is in fact a multistage process, only one portion of which uses light directly –Light versus dark reactions –Enzymes involved |
|
|
Discovery of Photosynthesis:
|
•C. B. van Niel (1897–1985)
–Found purple sulfur bacteria do not release O2but accumulate sulfur –Proposed general formula for photosynthesis •CO2+ 2 H2A + light energy → (CH2O) + H2O + 2 A –Later researchers found O2produced comes from water •Robin Hill (1899–1991) –Demonstrated Niel was right that light energy could be harvested and used in a reduction reaction |
|
|
Pigments
|
Molecules that absorb light energy in the visible range
•Light is a form of energy •Photon –particle of light –Acts as a discrete bundle of energy –Energy content of a photon is inversely proportional to the wavelength of the light •Photoelectric effect –removal of an electron from a molecule by light |
|
|
Absorption spectrum
|
When a photon strikes a molecule, its energy is either
–Lost as heat –Absorbed by the electrons of the molecule •Boosts electrons into higher energy level •Absorption spectrum –range and efficiency of photons molecule is capable of absorbing |
|
|
Pigments
|
Organisms have evolved a variety of different pigments
•Only two general types are used in green plant photosynthesis –Chlorophylls –Carotenoids •In some organisms, other molecules also absorb light energy |
|
|
Chlorophylls
|
Chlorophyll a
–Main pigment in plants and cyanobacteria –Only pigment that can act directly to convert light energy to chemical energy –Absorbs violet-blue and red light •Chlorophyll b –Accessory pigment or secondary pigment absorbing light wavelengths that chlorophyll adoes not absorb |
|
|
Bacterial Cell Division
|
•Bacteria divide by binary fission
–No sexual life cycle –Reproduction is clonal •Single, circular bacterial chromosome is replicated •Replication begins at the origin of replication and proceeds bidirectionally to site of termination •New chromosomes are partitioned to opposite ends of the cell •Septum forms to divide the cell into 2 cells |
|
|
Septation:
|
–Production of septum separates cell’s other components
–Begins with formation of ring of FtsZ proteins –Accumulation of other proteins follow –Structure contracts radially to pinch cell in 2 –FtsZ protein found in most prokaryotes –Shows a high degree of similarity to tubulin •Role in binary fission different from tubulin in mitosis |
|
|
Eukaryotic Chromosomes
|
•Every species has a different number of chromosomes
•Humans have 46 chromosomes in 23 nearly identical pairs –Additional/missing chromosomes usually fatal |

