![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
- 3rd side (hint)
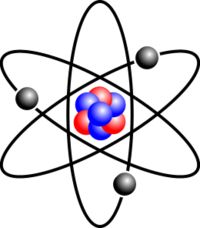
Atom
|
The smallest component of an element that still contains element properties
|
Small..... Contains element properties...
|
|
|
Molecule
|
Covalently bonded atoms. Smallest unit of a compound.
|
Compound's smallest _____... Composed of ________ bonded _____...
|
|
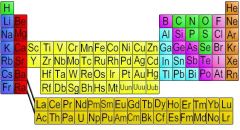
Element
|
Chemically identical substance made of atoms, and have an alike number of protons
|
Chemical, identical, composition of ____.
|
|
|
Proton
|
Particle in the nuclei of every atom, having a positive electrical charge.
|
+ charge, in the ____ of every atom
|
|
|
Electron
|
A particle that has varying numbers, is negatively charged and surrounds an atom's nucleus.
|
- charge, surrounds an atom's ____, varying ____...
|
|
|
Neutron
|
A particle that carries no electric charge and is subatomic
|
No electric _____ (something that is done to your phone every night)
|
|
|
Ion
|
An atom or molecule that has a positive or negative charge do to losing or gaining one or more electrons
|
- or + charge due to _____
|
|
|
Isotope
|
A form of an element having a different atomic mass but the same atomic number.
|
Different atomic ____ same atomic #
|
|
|
Ionic bond
|
Chemical bond formed by the attraction of oppositely charged ions.
|
Oppositely charged ions forming a ____ bond
|
|
|
Covalent bond
|
Two atoms sharing a pair of electrons creating a chemical bond.
|
_(#)? Atoms sharing a pair of ____ creating a ____ bond
|
|
|
Polar covalent bond
|
A bond in which electrons are shared between elements having a difference in electronegativity of between 0.5 and ~2.0.
|
Electrons shared between ___ having a difference in _____
|
|
|
Law of conversation of matter
|
Law that states matter can not be created or destroyed and does not hold true at the subatomic level
|
Subatomic level
|
|
|
Activation enery
|
The energy that you need to start a chemical reaction.
|
The ____ you need to start a ____ reaction.
|
|
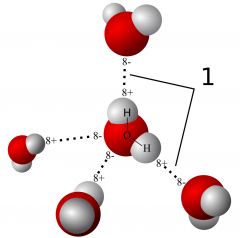
Hydrogen bond
|
A weak attraction between hydrogen atoms and oxygen, nitrogen, or fluorine atoms. It holds together the strands of DNA in their double helix.
|
Holds together strands if DNA
|
|
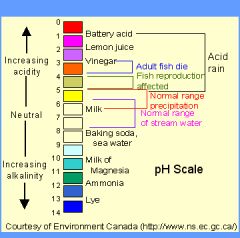
pH scale
|
A scale from 0 to 14 reflecting the concentration of hydrogen ions in solution
|
A number less than 7 denotes acidic conditions, and I number greater than 7 denotes basic conditions
|
|
Acid
|
Having a pH less than 7, having more dissolved hydrogen ions than hydroxide ions.
|
Less than 7... More ____ ions than ____ ions
|
|
|
Base
|
Having a pH greater than 7, having more dissolved hydroxide ions than hydrogen ions
|
Alkaline
|

