![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
76 Cards in this Set
- Front
- Back
|
Understand the challenges of vaccinating against respiratory pathogens
|
Upper Respiratory Tract (URT) is mainly IgA mediated whereas Lower Respiratory Tract (LRT) is mainly IgG mediated. Vaccines evoke a IgG response. IgA responses are not long-lived and difficult to obtain good secondary responses (repeated vaccine does not result in anamnestic response). IgA functions via immune exclusion- does not activate complement well
|
|
|
know advantages and disadvantages of various sample collection methods for the resp system and their interpretation
|
URT will contain normal flora. LRT should be essentialy sterile- contamination can occur during sampling though. Many URT commensals are LRT pathogens. LRT sample collection: o Transtracheal aspiration/wash
Contamination occurs if catheter travels cranially resulting in pharyngeal washing Most commonly done o Endotracheal collection methods Contamination is a problem o Bronchoalveolar lavage Guarded catheter helps avoid contamination A quantitive procedure that can be done “blind” or with an endoscope Contamination is a problem o Fine-needle lung aspirate Invasive procedure Very small sample Useful for focal abnormal area in lung in which a needle can be guided accurately with ultrasound guidance Potential to lacerate lung or vessel o Percutaneous lung biopsy Invasive procedure Very small sample Useful for focal abnormal area in lung in which a needle can be guided accurately with ultrasound guidance Potential to lacerate lung or vessel |
|
|
know the causative organisms of; symptomology; and predisposing factors for calf diphtheria and bullnose of swine
|
calf diphtheria: fusobacterium necrophorum- anaerobic gram neg rod. Loud inspiratory snore caused by trauma to the lining of the larynx and subsequent swelling. coughing/swallowing painful, calf will not eat/drink. Predispsing: dusty conditions, allergens, BRD. calves 3-8 months, feedlot animals.
bullnose of swine (necrotic rhinitis): fusobacterium necrophorum- anaerobic gram negatoive rod. Infections are characterized by necrosis and foul smelling exudate. Environmental pathogen |
|
|
know common predisposing causes of sinusitis in veterinary species
|
bacterial or fungal infections from dental dz (horses), URT viral infections (felines), trauma, tumors, dehorning in ruminants
|
|
|
understand the clinical signs, pathogenesis, and treatment of UR fungal infections
|
Clinical Signs/Pathogenesis
Epistaxis (Equines) Erosion of vessels (such as internal carotid artery) in guttural pouch Serosanguinous to hemorrhagic nasal discharge +/- cranial nerve signs (Equines) Sanguinous discharge (Canines) Epistaxis (Canines) Pain and ulceration of the external nares (Canines) Destruction of turbinate bones and local blood vessels (Canines) Treatment = Topical azole delivered as 1 hour infusion |
|
|
be able to ID aspergillus spp. Microscopically
|
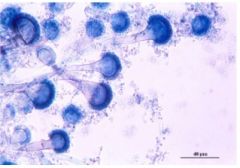
Philoconidia
Hyaline, septate hyphae that branch dichotomously |
|
|
know the causative agent(s) of equine guttural pouch empyema
|
streptococcus spp.
|
|
|
undersand the clinical signs, pathogenesis, diagnosis and tx of cryptococcosis and how it evades the host immune response
|
Clinical Signs: Granulomatous nasal disease, CNS disease (occasionally)
Pathogenesis: Inhalation of unencapsulated yeast cells form the environment; Nasal mucosa = primary site of infection; Lesions vary from expansive granulomas to little response; Capsulation of yeast in vivo Diagnosis Clinical Signs Impression smears of exudate or biopsy (KOH preps, Diff-Quik, India Ink) Culture Radiographs/CT= Thickened turbinates Serological testing (Latex agglutination test or antibody titers) Treatment (Excision/debulking, Antifungals = Polyenes with flucytosine or azoles) Capsule allows cryptococcus to evade the host immune system and provides protection. Very thick/wide capsule |
|
|
be able to ID cryptococcus microscopically
|
Encapsulated yeast with “narrow-necked” budding
|
|
|
know how cryptococcus differs from aspergillosis in clinical presentation
|
Aspergillosis = nasal atrophy, nasal deformity resolves with Tx
Cryptococcosis = Nasal thickening, no nasal deformity resolution with Tx |
|
|
know the origins of pleural infections and common causitive organisms
|
Pleural infections arise from:
Pneumonia Peritonitis migrating foreign body esophageal perforation hematogenous spread Common causative organisms" Actinomyces or Nocardia (Canines) Mixed anaerobes (Felines) Streptococcus, zooepidemicus, and anaerobes (Equines) |
|
|
•Know the microscopic appearance of streptococci and how they are differentiated from staphylococci
|
streptococci are gram positive, non motile cocci in pairs or chains that divide in one plane. They are catalase negative.
Staphylococcus are catalase positive |
|
|
•Know the reservoir, classification and hemolysis pattern of important Streptococcus spp.
|
s. agalactiae- ruminant mastitis, a hemolysis, group B
s. equi equi- strangles, b hemolsys, group c s. equi zooepidemicus- b hemolysis, gourp c s. dysgalactiae equisimilis- b hemolsyis, gorup c s. dysgalacatiae dysglaactaie, group c - ruminant mastitis entercoccus- a hemolsys, gorup d s. suis- zoonotic, group d, alpha hemoloysis s. porcinus- b hemoloysis, group E, s. canins- group G, b hemolysis |
|
|
•Know the pathogenesis of toxic shock syndrome and which streptococci cause it
|
• S. pyrogenes
• Cross links MHC II and T-cell receptor results in activation of T-cells in the absence of a specific peptide (antigen) that results in a cytokine cascade and multiorgan failure |
|
|
•Know the important Streptococcus spp. of animals, their Lancefield groupings and the diseases they cause
|
s. agalactiae- ruminant mastitis, a hemolysis, group B
s. equi equi- strangles, b hemolsys, group c s. equi zooepidemicus- b hemolysis, gourp c s. dysgalactiae equisimilis- b hemolsyis, gorup c s. dysgalacatiae dysglaactaie, group c - ruminant mastitis entercoccus- a hemolsys, gorup d s. suis- zoonotic, group d, alpha hemoloysis s. porcinus- b hemoloysis, group E, s. canis- group G, b hemolysis |
|
|
•Know the detailed pathogenesis, diagnosis, prevention and treatment of strangles and its sequelae
|
• Pathogenesis
o Enters the mouth or nose and attaches to cells in the crypts of the tonsil o Spreads to lymph nodes of pharyngeal areas as well as local pharyngeal and nasal mucosa o Does not survive within phagocytes but the capsule and M protein play a major role in preventing phagocytosis o Attraction of large numbers of neutrophils results in characteristic pyogenic response (have chemoattractant molecules in cell wall) o Most horses can effectively halt spread of the bacteria at the local lymph nodes • Diagnosis o Clinical signs and history o Microscopic exam of abscess aspirates o Culture • Prevention o Measures to prevent spread of disease through contact and fomites o vaccination Does not prevent infection Killed whole cell bacterin M protein extract Intranasal avirulent vaccine o Isolation of new horses on premises o Test potential carriers • Treatment o Isolate infected and exposed to horses o Penicillins are very effective Treat before clinical signs occur if exposed (Fever or anorexia) Do not treat if has lymph node swelling or abscessation unless dyspneic or signs of dissemination occur • Sequelae o Guttural pouch empyema +/- chondroids o Chronic carrier state o Purpura hemorrhagica—vasculitis; type 3 hypersensitivity reaction o “Bastard Strangles”—dissemination o Streptococcal immune-mediated myositis—muscle wasting |
|
|
•Know predisposing causes and common organisms found in pleural infections
|
Pleural infections arise from:
Pneumonia Peritonitis migrating foreign body esophageal perforation hematogenous spread Common causative organisms" Actinomyces or Nocardia (Canines) Mixed anaerobes (Felines) Streptococcus, zooepidemicus, a |
|
|
What are the Lancefield Groupings
|
Group A: Strep pyogenes
B: strep agalactiae C: strep equi, strep zooepidemicus, strep equisimilis, strep dysgalactiae D: enterococcus spp, strep suis E: strep porcinus G: Strep canis |
|
|
•Know the zoonotic and anthropozoonotic potential of streptococci
|
• S. suis—meningitis (humans)
• S. iniae • Group A streptococcal infections o S. pyrogenes o S. pneumoniae |
|
|
•Know the unique properties of the mycobacterial cell wall and its staining characteristics
|
• Rod with gram positive cell wall
• Will not stain with gram stain • Require acid-fast stains • More resistant to acids, alkalis, and disinfectants • Resistant to drying • Resistant to most commonly used antimicrobials |
|
|
•Know the groups of mycobacteria and important species within each
|
• Mycobacterium tuberculosis complex
o M. tuberculosis o M. bovis • Lepromatous mycobacteria o M. lepraemurium • Saprophytic mycobacteria o M. avium paratuberculosis o M. avium intracellulare complex |
|
|
•Know the pathogenesis of tuberculosis
|
• Enter via respiratory tract/GI tract
• Phagocytosis by alveolar macrophages and survive within o Secretes enzymes that inactivate phagosome-lysosomal enzymes • Infection draining lymph node, possibly disseminate, and local spread of infection • T-cell mediated immunity (some activated macrophages can kill the mycobacterium) • Lesions represent host cell-mediated response (granuloma) |
|
|
•Know animal species commonly affected by important mycobacterial species
|
• M. bovis (Bovine)
• M. avium paratuberculosis (Bovine) • M. lepraemurium (Felines) • M. avium intracellulare complex (Canines, Felines, and Avians) • M. tuberculosis (Canines and Psittacine birds) |
|
|
•Understand the basics of the US animal tuberculosis test and eradication program
|
• Major tool is intradermal skin testing
|
|
|
•Know how tuberculosis is diagnosed, including skin testing methods
|
• Demonstration on exudates, aspirates, or biopsy
• Intradermal skin testing o Inject intradermally with Protein purified derivative of M. bovis o Wait 72 hours. Then, read. Any reaction by feeling or visualizing is positive. o If any reaction, call state vet |
|
|
•Know in general terms when and how tuberculosis is treated in animals
|
• No treatment in LA = Slaughter
• Long term and expensive in SA = Not recommended o At least 2 antibiotics used and not typically used in practice |
|
|
•Know the age group and species primarily affected by Rhodococcus equi
|
• Foals—2weeks -7 month with most 4-10 weeks
|
|
|
•Know the reservoir of Rhodococcus and how it is propagated and transmitted (including seasonal influences)
|
• Inapparent carrier mares
• Saprophyte multiplies dramatically in horse manure and soil contamination with horse feces • Highest incidence in summer because of dust and susceptible population • Transmitted by inhalation of organism on dust particles • Ingestion can occur in a GI form (less common) |
|
|
•Know the clinical signs and pathogenesis of the disease and what extrapulmonary manifestations occur
|
• Pathogenesis
o Infection occurs in the first week after birth by inhaling organism and depositing in LRT o Macrophages in foals are unable to kill the organism due to failure of phagosome-lysosome fusion o Forms pyogranulomatous bronchopneumonia and granulomas in lung o Hematogenous spread can occur • Clinical signs o Uveitis o Diarrhea o Synovitis o pyogranulomatous bronchopneumonia and granulomas in lung |
|
|
•Be able to describe the role of the Vap plasmid
|
• Virulence factor; no plasmid = no disease
• Encodes for 8 virulence proteins with different virulence factors for each |
|
|
•Know what methods of prevention and early detection are in use
|
• No vaccines
• Administration of hyperimmune plasma • Screening at risk foals with daily temperatures and biweekly fibrinogen and/or thoracic ultrasound |
|
|
•Know what non-equine species are affected by Rhodococcus and how the disease manifests in those species
|
• Swine, bovine, caprine, and llamas—pyogranulomatous to necrotizing lymphadenitis
• Felines—ulcerative and pyogranulomatous dermatitis (infrequent occurrence) |
|
|
•Know what Bordetella organisms cause disease in veterinary species, and know the reservoir and mode of transmission of Bordetella
|
• B. bronchiseptica—Canines, Felines, Swine, and lab animals
• B. avium—turkeys • Reservoir is Canine, Felines, Swine, and llagomorphs • Transmitted by direct contact or aerosol droplets |
|
|
•Understand the clinical syndrome of Infectious Tracheobronchitis (Kennel Cough) and know what organisms are involved
|
• Clinical signs—paroxysmal harsh, dry cough; retching; gagging; secondary pneumonia;
• B. bronchiseptica in combination with viruses (canine parainfluenza virus and canine infectious laryngotracheitis virus (CA2)) |
|
|
•Know the pathogenesis and important virulence factors and exotoxins of Bordetella bronchiseptica, and know how the immune system responds to Bordetella infection
|
• Pathogenesis
o Viral infection inhibits respiratory defense mechanism which predisposes infection to B. bronchiseptica o B. bronchiseptica attaches to cilia of URT epithelium via adhesins found on the outer membrane and via fimbriae o Bordetella LPS protects against complement and defensins ( O antigen is very long and serves as adhesins in addition to protecting against complement and defensins o Encapsulation enables evasion of phagocytosis o Remains on surface of epithelia l cell to proliferate and produce 4 exotoxins Tracheal cytotoxin induces ciliostasis and destruction of the epithelial cells Adenylyl cyclase inhibits phagocytosis by macrophages and also inhibits the respiratory burst of neutrophils o Bacterial growth in the tracheal lumen results in attraction of PMNs to the mucosal surface which results in accumulation of mucus and neutrophilic exudate in bronchi and trachea o Elimination takes 6-14 weeks o IgA= main defense but only prevents bacterial attachment and toxin neutralization |
|
|
•Know the risk factors for development of Bordetella pneumonia
|
• Co-infection with distemper
• Puppies |
|
|
•Understand the factors involved in choosing to treat Bordetella infection and know how Bordetella infection may be controlled
|
• Control
o Isolation o Cleaning of premises o Vaccination • Bordetella treatment o No resistance reported o Self-limiting disease (takes a long time) o Treat more for owner than patient o Antimicrobial therapy does not diminish or shorten duration of clinical signs o Always treat puppies with antibiotics o If suspect systemic infection or pneumonia, treat with antimicrobials |
|
|
•Know what viruses are involved in bordetellosis of cats
|
• Feline herpesvirus-1
• Feline calicivirus |
|
|
•Know the manifestations of Bordetella infection in mammalian species other than dogs and cats
|
• Swine: secondary pneumonia and atrophic rhinitis
• Guinea pigs: pneumonia • Rabbits: carrier; pneumonia; |
|
|
•Know the etiologic agent and clinical signs of turkey rhinotracheitis
|
• Bordetella avium
• Turkeys: Rhinotracheitis; decreased appetite, weight gain, and feed efficiency; loss of voice; ocular and nasal discharge; conjunctivitis; sneezing; “snicking” and flicking the head; dyspnea |
|
|
•Know the USA geographic distribution of histoplasmosis, blastomycosis and coccidioidomycosis
|
• Southwest= coccidioidomycosis
• Central to East Central = Histoplasmosis • East to Central = Blastomycosis |
|
|
•Describe the characteristic lesions of each disease (histo/ blast/coccidioidomycosis)
|
• Granulomatous inflammation to pyogranulomatous inflammation
|
|
|
•Know the etiologic agent and disease pathogenesis for each disease (histo/ blast/coccidioidomycosis)
|
• Histoplasmosis—Histoplasma capsulatum
o Pathogenesis Inhalation of microconidia from environment Sporulates and converts to yeast phase in vivo Replication of yeast phase in monocytic cells (usually macrophages) • Organisms bind to phagocytes via adhesins • Adhesin-integrin binding diminishes respiratory burst • Organism decreases phagolysosome pH • Multiply within macrophage then lyses cell May spread from lungs via blood or lymphatics to GI tract, liver, spleen, and/or bone marrow Resolution requires cell-mediated immunity • Blastomycosis—Blastomyces dermatitidis o Pathogenesis Inhalation of conidia from environment converts to yeast phase in vivo Adhesin Bad1 allows phagocytosis without respiratory burst and down regulates TNF-alpha Grows intracellularly in macrophages and giants cell and extracellularly as large budding yeast May spread from lungs via lymphatics and/or blood to skin, eyes, bones, and joints • Coccidioidomycosis—coccidioides immitis o Pathogenesis Inhalation of arthroconidia from soil Development of spherule from arthroconidium Spherule matures and produces endospores that are released in vivo (or in the lung) May localize to foci within the lung, extend to lymph nodes, or disseminate Bone and skin are tissues in which dissemination most often occurs |
|
|
•Be able to describe the characteristic clinical signs for each disease (histo/ blast/coccidioidomycosis)
|
• Histoplasmosis—granulomatous lesions; increased respiratory rate; increased respiratory effort; cough (not in cats); anemia (cats); inappetence; weight loss; fever; poor response to antibiotics; internal lymph node involvement; perfuse diarrhea (chronic);
• Blastomycosis—depression; fever; weight loss; poor response to antibiotics; generalized lymphadenopathy; ulcerative skin lesions; ocular and bone involvement; lame dog (chronically); hunting dogs have a pre-disposition; pyogranulomatous lesions; • Coccidioidomycosis—fever; anorexia; weight loss; poor response to antibiotics; lameness; draining skin lesions; ocular infection; pericarditis; |
|
|
•Know how to diagnose each disease (histo/ blast/coccidioidomycosis)
|
• Histoplasmosis
o Clinical signs and history o Serology (not very good) o Demonstration of agent in tissues or samples o Culture by qualified lab • Blastomycosis o Clinical signs and history o Demonstration of the agent in tissues or sample (Demonstration = Diagnosis) o Serology o Culture by qualified lab • Coccidioidomycosis o Clinical signs and history o Demonstration of organism (Demonstration = disease) o Serology (Dissemination can result in no detectable antibodies b/c of anergy) o Culture by qualified lab |
|
|
•Understand the basics of treatment for each disease (histo/ blast/coccidioidomycosis)
|
• Coccidioidomycosis
o Azoles—long term for 8-12 months minimum o Amphotericin B (toxic) • Blastomycosis o Azoles—2-3 month treatment minimum (Best = itraconazole) o Amphotericin B for severe disease (toxic) • Histoplasmosis o Azoles—long term treatment for 4-6 months minimum (Itraconazole or posaconazole) o Amphotericin B in combination with oral azole drug if severe disease o Anti-inflammatory drugs |
|
|
•Recognize the causative agents microscopically, both in tissue and in culture, and be able to describe them (Coccidioidomycosis, histo, blasto)
|
• Histoplasmosis
o Culture Mold characterized by hyaline hyphae and development of microconidia and large, thick walled tuberculate macroconidia (room temperature) Small budding yeast cells (body temperature) o Tissue Small yeast cells within macrophages or neutrophils Round yeast with basophilic center and a clear halo Much smaller than cryptococcus yeasts • Blastomycosis o Culture Mold characterized by hyaline hyphae and development of spherical conidia that appear as balloons on short stalks (room temperature) Spherical, broad-based with thick cell wall (body temperature) o Tissue Spherical, thick-walled fairly large broad-based budding yeast cells Usually plentiful Non-encapsulated • Coccidioidomycosis o Culture Mold characterized by hyaline hyphae and development of alternating, barrel-shaped arthroconidia (room temperature and body temperature) o Tissue Very large round double-walled spherules Viewed unstained or with PAS or Papanicolaou stain |
|
|
•Be familiar with pneumocystosis and know the animals usually affected
|
• Uncommon; Opportunistic; Miniature dachshunds < 1 year old and Foals esp. foals with SCID; associated in immunocompromised animals; not zoonotic; Pneumocystis carinii is found in a cyst and trophozoite stage;
|
|
|
•Provide a BRIEF overview of Family Pasteurellaceae
|
• Family of gram-negative coccobacilli
• Many are commensals in respiratory, gastrointestinal, and/or reproductive tracts of mammals and birds |
|
|
•Characterize pathogenic bacteria of genera Pasteurella, Mannheimia, & Bibersteinia.
|
• Gram-negative rod (coccobacillus)
• Found as commensals on mucous membranes especially of the oropharyngeal, nasopharyngeal, and tonsilar areas • Opportunistic pathogens associated with disease in: o Cattle o Sheep and goats o Pigs o Birds o Dogs and Cats (sporadically) o Not in horses |
|
|
•Discuss pathogenesis, clinical signs, lesions, treatment and prevention of those bacteria in rabbit respiratory dz
|
snuffles in rabbits. Often assoc with stress. Major cause of: mucopurulent rhinosinusitis, pneumonia, otitis media/interna, purulent infection in numerous tissues, septicema. Difficult to control- most rabbits are carrriers. Lesions include bronchopneumona, fibrinous pleuropneumonia, pulmonary abscesses. Also head tilt, conjunctivitis with mucupurulent ocular discharge, cutaneous abscesses
|
|
|
•Discuss pathogenesis, clinical signs, lesions, treatment and prevention of those bacteria in Fowl cholera
|
o A systemic and respiratory infections of chickens, turkeys, and waterfowl
o Peracute disease results in sudden death o Acute septicemia o Lesions in a variety of organs o Acute death o Randomly scattered petechial hemorrhages in heart and GI tract or multifocal small foci of hepatic necrosis o Respiratory disease often manifest as a chronic sinusitis and or synovitis Sinusitis—severe unilateral or bilateral swellings caudal to the beak due to accumulations of caseous exudate o Pasteurella multocida Septicemia/respiratory disease o Control Biosecurity and sanitation Vaccinations—incomplete protection Treatment—last resort and check sensitivity of isolates |
|
|
•Discuss pathogenesis, clinical signs, lesions, treatment and prevention of those bacteria in Ruminant respiratory disease
|
o Mannheimia haemolytica (Pasteurella haemolytica Biotype A)
Acute, severe pleuropneumonia in cattle goats, and sheep Mastitis (ewes) Cause of ACUTE cases of shipping fever o Shipping Fever/BRD Causative Agent Isolates • Mannheimia haemolytica • Histophilus somni (Haemophilus somnus) • Pasteurella multocida • Bibersteinia trehalosi • Arcanobacterium (Actinomyces) pyogenes (Chronic cases) • Mycoplasma Bovis (Subacute to Chronic cases) Classic pleuropneumonia/bronchopneumonia Severe fibrinous pleuropneumonia or bronchopneumonia • Found in stressed cattle Susceptible • Pre-weaning dairy calves < 3 months • Post-weaning beef calves from 6-18 months of age • Stressful conditions o Concurrent viral infection o Weaning o Co-mingling (very stressful) o Shipping o Inclement weather Most common disease of beef cattle Pathogenesis • Requires immunocompromised host due to stress and/or virus infection that decrease the innate and adaptive immune mechanisms of the respiratory tract and pathogenic bacteria capable of causing pneumonia • M. haemolytica serotype 1 is part of the normal nasopharyngeal flora of calves that is usually found in the tonsils • Stressing the calves results in proliferation of M. haemolytica and inhaled in the lung • Virulence factors o Capsule—anti-phagocytic o Leukotoxin (LKT)—toxic to ruminant macrophages and neutrophils o Endotoxin—damages vessels walls Major clinical signs • Nasal discharge; dyspnea; cough; anorexia; abnormal lung sounds; fever; stand with neck extended and feet spread apart stance • Chronic pneumonia—lack of weight gain; lethargy; anorexia; chronic respiratory signs; Lesions—fibrin-rich fluid in pleural cavity, on pleural surface, and distending interlobular septa; prominent coagulation lobular necrosis; Control/Prevention • Control stressors • Pre-condition programs o Wean and hold for 30-45 days before sale o Anthelmintics o Vaccinations • Avoid co-mingling and sale barns • Decrease transport time • Decrease crowding • Good nutrition • Viral vaccines—reduce but do not prevent • Bacterial vaccines—Enhance productivity 50% of time Treatment • Antibiotics • Early recognition and treatment—mandatory • Resistant to older antimicrobials o Enzootic Pneumonia of Dairy Calves Respiratory disease complex of young dairy calves usually less than 3-4 months of age 2nd most common disease of dairy cattle Purulent bronchopneumonia Stress factors • Failure of Passive Transfer (FPT) • Nutritional Deficiencies • Adverse environmental conditions Causative agents • Bovine Respiratory viruses—predispose to bacterial infection and pneumonia • Pasteurella multocida Serogroup A—most common isolate Control • Eliminate predisposing factors o Adequate colostrum form immunized dams o Hygiene o Appropriate air flow o Adequate diet o No good vaccines available Treatment • Antimicrobials o Bibersteinia trehalose (Pasteurella haemolytica Biotype T and Pasteurella trehalosi) Ruminants only Septicemia in lambs (winter months) Pneumonia (free ranging bighorn sheep) Emerging pathogen in bovine pneumonia o Pasteurella multocida Found in nasopharynx and/or oropharynx of a variety of animals: ruminants, swine, dogs, and cats Suppurative bronchopneumonia |
|
|
•Discuss pathogenesis, clinical signs, lesions, treatment and prevention of those bacteria in Swine upper respiratory disease
|
o Pasteurella multocida
Found in nasopharynx and/or oropharynx of a variety of animals: ruminants, swine, dogs, and cats Sporadic cause of suppurative bronchopneumonia Atrophic rhinitis • Disease of young pigs in which infection usually occurs by 3 weeks of age • Atrophy of nasal turbinate bones • Clinical signs—sneezing; lacrimation; distortion of the snout; • Severe, chronic atrophic rhinitis results in nearly complete turbinate atrophy and lateral deviation of the snout o Usually affects ventral scrolls • Pathogenesis o B. bronchiseptica colonizes nasal mucosa and produces a toxin that inhibits the osteoblasts of the nasal turbinates o This enhances colonization of toxigenic strains of P. multocida that produces Pasteurella multocida toxin that enhances bone resorption (increased osteoclast activity) leading to turbinate loss • Control o Antibiotics that reduce bacteria levels in both sows and piglets o Management procedures Improve air conditions Use all-in and all-out housing o Vaccination program using bacterin toxoids |
|
|
•Describe zoonotic potential
|
• P. multocida is an oral/pharyngeal commensal in dogs and cats. Bite wounds can become infected and lead to septicemic in neonates and HIV patients. May also be obtained from dog kissing and cat cuddling.
|
|
|
•Know the characteristics of mycoplasmas
|
• smallest free-living bacteria
• Lack a cell wall and peptidoglycan • typically colonize mucous membranes • Have a very small genome • Lack genes to form a cell wall • Extracellular organism but require intimate assoc. with host cells • Resistant to beta-lactam antibiotics • Pleomorphic • Filterable • Fried-egg colony morphology (umbonate) • Have complex nutritional requirements for in vitro growth |
|
|
•Know the difference between mycoplasmas and L-form bacteria
|
• L-form bacteria are cell wall deficient that can turn their cell wall off (have the genetic capability to form a cell wall) to evade the immune response. External conditions inhibit cell wall synthesis but allow the bacterium to survive (not lyse). L-form bacteria are rare in clinical situations.
|
|
|
•Know the reservoirs and mode of transmission of mycoplasmas
|
• Reservoir is mucous membrane of animals such as URT or lower genitourinary tract
• Transmission is via airborne droplets, direct contact, venereal, and egg transmission in poultry |
|
|
•Know the demographics and disease manifestations of mycoplasmas in common veterinary species
|
• Usually chronic diseases with low mortality. In other words, they are economic diseases associated with confinement raised and intensively reared animals
• Disease manifestations are respiratory disease, arthritis/synovitis, mastitis, keratoconjunctivitis, and reproductive tract infections that are more common in younger animals |
|
|
•Be familiar with the general pathogenesis of mycoplasmas
|
• Not well undersold
• Close association with respiratory epithelial cells • High ammonia in the air enhances Mycoplasma infection • Ciliostasis (some infections) • Many spp. have variable surface proteins (VSPs) that change antigenic structure to allow for evasion of the immune system • May damage host cells by release of factors (proteases, H2O2,and hemolysins) |
|
|
•Know which mycoplasmas are reportable agents
|
• Mycoplasma gallisepticum
mycoplasma mycoides mycoides small colony |
|
|
•Know the major mycoplasmas affecting swine, poultry, cattle, sheep, goats, small animals and horses, and their disease patterns
|
• Swine
o M. hyopneumoniae—pneumonia o M. hyorhinis—polyserositis and arthritis o M. hyosynoviae—arthritis • Poultry o M. gallisepticum—respiratory disease o M. meleagridis—air sacculitis with synovitis o M. iowae—air sacculitis o M. synoviae—infectious synovitis • Cattle o M. mycoides mycoides (small colony)—fibrinous pleuropneumonia o M. bovis—pneumonia with arthritis and mastitis o M. bovirhinis—upper respiratory commensal o M. dispar—pneumonia ( predominantly in Europe) o M. californicum—Bovine mastitis o M. canadense—Bovine mastitis o M. bovigenitalium and Ureaplasma diversum—Granular vulvovaginitis • Sheep o M. agalactiae—contagious agalactia o M. conjunctivae—keratoconjunctivitis o M. ovipneumoniae—role unclear • Goats o M. agalactiae—contagious agalactia o M. conjunctivae—keratoconjunctivitis o M. mycoides mycoides (large colony)—pneumonia accompanied by septicemia, arthritis, and mastitis o M. capricolum capricolum—acute interstitial pneumonia with septicemia o M. capricolum capripneumoniae—fibrinous pleuropneumonia • Dogs o M. cynos—pneumonia o M. canis—urogenital tract disease o M. spumans—arthritis • Cats o M. felis—conjunctivitis o M. gatae—arthritis • Horses o M. felis—pleuritis |
|
|
•Know control measures for Mycoplasma hyopneumoniae
|
• Treatment with macrolides or tetracyclines (if enzootic to a herd)
• Vaccination programs—decrease clinical signs and gross signs • Management procedures—decrease overcrowding; enhance air-quality; all-in all-out (AIAO) procedures ; • Establishment of M. hyopneumoniae-free swine herds |
|
|
•Know the etiologic agent, lesion and reservoir for CBPP
|
• Mycoplasma mycoides mycoides (small colony)—causative agent
• Lesion—severe necrotic fibrinous pleuropneumonia • Reservoir—chronically infected animals |
|
|
•Know the role of Mycoplasma bovis in feedlot pneumonia and its lesion
|
• Subacute to chronic pneumonia in feedlot cattle
• Lesion—fibrinous synovitis; multiple small coalescing foci of caseous pneumonia |
|
|
•Know the etiologic agent, characteristics and growth requirements for porcine pleuropneumonia
|
• Etiologic agent—Actinobacillus pleuropneumoniae (Haemophilus pleuropneumoniae)
o Gram-negative coccobacillus o V-factor dependant (NAD) |
|
|
•Know the reservoir, mode of transmission and virulence factors for Actinobacillus pleuropneumoniae
|
• Reservoir—swine such as a carrier pig
• Transmission—aerosol droplet; direct contact • Virulence factors o polysaccharide capsule o Cytotoxins—toxic to phagocytic cells o Urease—increases phagolysosome pH and activates phagocytes o Endotoxin (LPS)—increased vascular permeability and vessel thrombosis in lung; edema, hemorrhage, and necrosis in lung |
|
|
•Know the clinical signs, disease patterns and necropsy lesions of Actinobacillus pleuropneumoniae
|
• Clinical signs—sudden death (peracute); fever, respiratory distress, open-mouth breathing, blood-stained froth from nostrils and mouth (acute); cough and poor weight gains (chronic);
• Disease patterns o Peracute—sudden death; Necropsy lesions—severe fibronecrotic and hemorrhagic pneumonia with fibrinous pleuritis Endotoxin—major role in the disease o Acute—fever, respiratory distress, open-mouth breathing, blood-stained froth from nostrils and mouth o Chronic—cough; poor weight gains; Sequestered lesions and/or pleuritis at slaughter; • Necropsy lesions—sequestered lesions and/or pleuritis at slaughter; chronic focal abscesses and associated pleuritis; severe fibrinonecrotic and hemorrhagic pneumonia and fibrinous pleuritis; |
|
|
•Know how porcine pleuropneumonia is diagnosed and viable control methods
|
• Diagnosis—clinical signs; necropsy lesions; definitive diagnosis via culture; serology;
• Control—difficult; All-in All out management; vaccines; |
|
|
•Be familiar with the characteristics and disease patterns of Trueperella pyogenes
|
• Small gram-positive rod that is a commensal on mucous membranes of ruminants and swine involved in numerous pyogenic infections, secondary pneumonia, abscesses, and reproductive tract infections
|
|
|
•Know the disease-causing organisms in family Chlamydiaceae, the affected species and disease patterns
|
• Chlamydia trachomatis
o Conjunctivitis and reproductive tract infections (STD) o Humans • Chlamydia suis o Subclinical o Conjunctivitis, enteritis, or pneumonia o Swine • Chlamydia muridarum o Subclinical respiratory infections o Mice and hamsters • Chlamydophila psittaci o psittacosis and ornithosis o Birds—parrots and psittacine birds are at high risk • Chlamydophila felis o Feline o Conjunctivitis • Chlamydophila caviae o Guinea pig o Conjunctivitis • Chlamydophila pneumoniae o Respiratory pathogens o Humans o Koalas o Horses • Chlamydophila pecorum o Respiratory and urogenital tract disease in koalas o Abortion, conjunctivitis, encephalomyelitis, enteritis, and pneumonia and poly arthritis in ruminants and swine • Chlamydophila abortus o Abortion in ruminants Especially sheep and goats |
|
|
•Know how chlamydial organisms are transmitted
|
• Inhalation or ingestion of the elementary bodies in dried fecal material or nasal exudate
|
|
|
•Know the life cycle of Chlamydophila psittaci
|
• Elementary body (infectious agent) attaches to host cell and initiates endocytosis
• Elementary body in phagosome inhibits lysosome fusion with phagosome • Binary fission occurs within phagosome o Lost ability to produce energy and use ATP from host “energy parasites” • Binary fission products (cells) develop into elementary bodies which are released by cell lysis or exocytosis (possibly) |
|
|
•Know the pathogenesis of Cp. psittaci infection
|
• Entry into host cells (variety of cell types can be infected) occurs on mucosal surfaces
• Bacteremia may occur resulting in infection in a variety of tissues • Damage is thought to be due to host cell lysis • Disease syndromes are related to the infecting chlamydial species and strain as well as the host species • Chronic or persistent infections without clinical signs are common • Some infections may be latent • Active infections are sometimes stress-related |
|
|
•Know the clinical signs of generalized avian psittacosis and how the disease may be diagnosed and treated
|
• Clinical signs
o Lethargy o Anorexia o Ruffled feathers o Serous to mucopurulent oculonasal discharge o Greenish diarrhea o Decreased egg production o Emaciated, Dehydrated, and Die • Diagnosis o Impression smears o Culture o Serology o Antigen tests • Treatment o Isolate the bird o Treat with tetracycline or doxycycline for 45 days o Keep cage litter, feathers, and any aerosolized or possibly contaminated material to a minimum o Susceptible to most disinfectants (QUATS and Bleach (1:100) |
|
|
•Know the zoonotic potential of psittacosis and protective measures to be taken
|
• Persons cleaning or handling infected cages should wear protective clothing, gloves, disposable surgical cap, and a fitted respirator or N95 mask
• At necropsy, wet bird with disinfectant, wear protective equipment and work in a safety cabinet (or send to OADDL) • Humans are usually infected by inhalation of elementary bodies in dried, infective bird feces • Clinical signs are often flu-like and may include: fever, headache, anorexia, sore throat, and sometimes pneumonia |

