![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
55 Cards in this Set
- Front
- Back
|
What determines the chemical properties of Atoms?
|
The number of electrons; in electrically neutral atoms, #of electrons =Z
|
|
|
What is Z?
|
Z=Number of protons
|
|
|
What is the Pauli exclusion principle?
|
The maximum number of electrons allowed for an inner shell = 2n^2
K=1; 2 electrons L=2; 8 electrons M=3; 18 electrons |
|
|
What is the binding energy of an electron?
|
Energy required to remove an electron from an atom
|
|
|
Is electrical force directly or indirectly proportional to distance
|
Electrical force is inversely proportional to the square of the distance between two charged particles; 1/d^2
K shells are the most 'tightly bound.' Binding energy increases with larger Z values (larger nuclear charge) |
|
|
What are the 2 possible outcomes when energy is imparted on an atom's electron?
|
1) energy is stong enough to overcome binding energy of inner shell electron which creates a vacancy (ionization)
2) energy is not strong enough, electrons may jump to an outer shell electron and holds the energy (excitation) |
|
|
What are the 2 possible outcomes of an excited electron?
|
1) Release of "characteristic x-ray"
2) Release of "Auger Electron" |
|
|
What is a 'characteristic x-ray'?
|
Xray photon released released when an excited outer shell electron fills the vacancy of an inner shell electron.
Energy is equal to difference in binding energy between the 2 shells. |
|
|
What is an 'Auger Electron'?
|
Outer shell electron released when excited inner shell electron falls to its ground state. No x-rays are released in this process.
|
|
|
Are heavy elements more likely to generate characteristic xrays or Auger electrons?
|
Heavy elements: Characteristic xrays
Light elements: Auger electrons |
|
|
What is a A?
|
A = mass number= number of protons + neutrons
(aka: number of 'nucleons') Protons carry a +charge Neutrons are neutral |
|
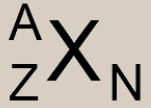
Atomic Nomenclature: Define Variables
|
X= Element symbol; defined by Z:
A= Mass number Z= Atomic number N= Number of Neutrons = A-Z |
|
|
What are the opposing forces in a nucleus
|
Repulsive forces between protons
Attractive forces between nucleons (protons and neutrons) |
|
|
What is the half life of Tc99m
When it decays, how is the energy released? What is the energy released? |
6hrs
gamma radiation 140.5 keV |
|
|
What are isotopes
|
isotoPe= same number of Protons = same Z
|
|
|
What are isobars
|
isobAr= same atomic mass number = same A
|
|
|
What are isotones
|
isotoNe= same number of Neutrons = same N
|
|
|
What are isomers
|
isomer= same composition but different energy= same N and Z.
|
|
|
What is line of stability
|
The N/Z ratio needed to maintain nuclear stability.
For low Z elements N/Z=1 For high Z elements N/Z approaches 1.5 Rationale: Attractive forces occur between protons:neutrons and neutrons:neutrons. As repulsive forces increase (Z), more neutrons are necessary to maintain attraction. |
|
|
What is gamma decay?
|
An excited nucleus transforms to a more stable lower energy state by emitting the extra energy as a gamma photon. No change in composition of nucleus (therefore called ISOMERIC transition)
|
|
|
What is B- decay?
Does this occur in a neutron-rich or neutron-poor nucleus |
Occurs in neutron-rich nucleus
N-> P + e- + V(antineutrino) Net conversion is neutron to proton. |
|
|
What is V(antineutrino)?
|
antiparticle that carries energy but neither mass nor charge.
|
|
|
How does the mass number change after B- decay
|
It doesn't. The total number of nucleons are unchanged.
|
|
|
How does the atomic number change after B- decay
|
Z+1
|
|
|
What's the difference between the free electron emitted in B- decay and the electron released from internal conversion?
|
B- decay electron is released from the nucleus (and have a continuous energy spectrum)
internal conversion electron is an orbital electron (and caries a discrete series of energies) |
|
|
What form of radioactive decay is used to PRODUCE
Tc99m What is the formula? |
B-decay
99Mo-> 99mTc +e- + V(antinutrino) |
|
|
How does 99mTc decay?
What are the 2 possible release products? |
gamma decay
99mTc->99Tc + gamma photon (or conversion electron) The gamma photon contributes to image formation The conversion electron will be completely absorbed by the patient. |
|
|
What is electron capture?
What is the net effect? This results in a similar effect on the nucleus to what other form of decay? |
An orbital electron is "captured" by the nucleus, combines with a proton to form a neutron.
Z-1 Same net result as B+ decay |
|
|
With respect to B+ decay and Electron capture, which occurs among heavier elements and lighter elements
|
Heavy elements= electron capture
light elements= B+ decay rationale: electrons are closer to nucleus in heavy elements, increasing likelihood of "capture" |
|
|
What happens to the vacancy in the electron shell after electron capture?
|
Vacancy is filled by an outer shell electron and produces either a characteristic xray or Auger electron.
|
|
|
What is internal conversion?
What happens to the electron if the energy released can overcome the binding energy. |
Alternative to gamma decay where energy is transferred to orbital electron.
It it released from the atom and is termed a conversion electron. |
|
|
Which orbital electrons are most likely to participate in electron capture, or internal conversion?
|
Inner shell, K and L
|
|
|
What is B+ decay
What is the formula What is the net effect on the nucleus |
P->N + e+ +V(neutreno)
Z-1 |
|
|
Does beta decay occur in proton-rich or proton-poor excited nuclei
|
Proton-rich
|
|
|
What happens to the positron released during B+ decay?
|
combines with an electron in the environment to undergo annihilation.
|
|
|
What are the products of annihilation
|
Two 511keV gamma rays which travel in opposite directions
|
|
|
What is the prototypic imaging modality based on B+ decay?
What is the nuclide? What is the formula? |
PET imaging
F18 18F ->18O +e+ + Neutreno e+ + e- -> 2 gamma photons |
|
|
What is alpha decay/ alpha particle
|
Nucleus ejects alpha particle (2 protons and 2 neutrons)
same as 4He nucleus |
|
|
What is the only form of radioactive decay that alters the atomic mass?
|
alpha decay
|
|
|
What is nuclear fission?
What are the products? |
Heavy nucleus spontaneously breaks into 2 lighter nuclei
|
|
|
What is the formula to measure radioactivity (A)?
What is the SI unit of decay? What is the Traditional unit of decay? How are they related? |
A(t)=-dN/dt
SI unit= bacquerel (Bq) = 1decay/sec Traditional unit= Curie (Ci)= 3.7X10^10 decay/sec 1mCi=37 MBq |
|
|
Radioactivity Example:
N(0)=1000 decay constant 0.1/sec= 10%/sec how many radioactive nuclei remain after 3 sec? |
729
|
|
|
What is physical half life?
What is the equation? |
Time it takes to decrease the number of radioactive nuclei by 1/2
0.693/(lambda) decay constant |
|
|
What is average life?
What is the formula |
The average life of a large quantity of nuclei
1.44 x T1/2 |
|
|
What is effective half life (Te)?
What is the formula? |
Life of a radionuclide in a patient that factors physical half-life (Tp) and biologic half-life (Tb)
Te=TpTb/(Tp+Tb) The product divided by the sum |
|
|
What is parent-child-grandchild decay?
|
An unstable parent nuclide decays to a child nuclide which may not be stable. This will undergo another decay to produce a grandchild nuclide.
example 99mTc 1. 99Mo->99mTc +e- + V(antinutrino) 2. 99mTc->99Tc +2 gamma photons (or conversion electrons) The decay of the child is dependent on the both the half lives of the parent (Tp) and child (Tc) |
|
|
What pattern of equilibrium occurs when the half life of the parent is long (approaches infinity)
Tp>>>Tc |
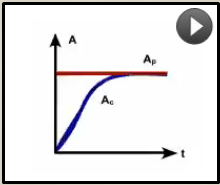
Secular equilibrium
Ac will increase and by 5 child half live (Tc) will =Ap Example (226Ra) |
|
|
What pattern of equilibrium occurs when the half life of the parent is longer than the child but not that long?
Tp>Tc |
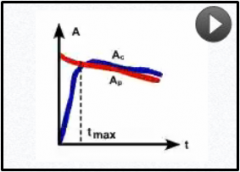
Transient equilibrium
Ac will peak and then decline at the same rate of Ap Ex: 99mTc: Peaks by 24hrs then degrades Why we whould extract from generator once/day for maximal activity |
|
|
What pattern of equilibriium occurs when half life of the child is longer than the parent?
Tc>Tp |
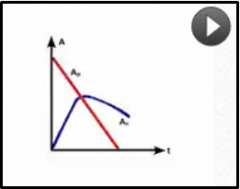
No equilibrium,
Ac will peak and decline at a rate with no relationship to the parent activotu |
|
|
T1/2 example:
If a radionuclide decays at 1%= lambda=0.01/day), how long will it take to decay to half of its original activity |
70 days
T1/2= 0.693/lambda |
|
|
Effective halflife example:
If the biological and physical half lives of a radionuclide are both 3hrs, the effective half life is how long? |
1.5 hrs
Te=TpTb/(Tp+Tb) |
|
|
The binding energy of an electron depends one what?
|
distance from nucleus.
Inner shell electrons have higher binding energies than outer shell electrons |
|
|
B+ decay example:
In B+ decay of potassium to 40Ar18, A, N and Z of potassium are: |
A=40
Z=19 |
|
|
How may half lives must pass for the radioactivity to decay to 10%
|
3-4 half lives
|
|
|
What is the half life of 99mTc?
|
6 hours
|

