![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
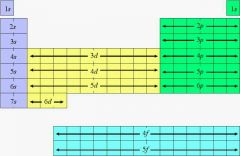
Periodic table according to valences |
|
|
|
|
Energy level |
Specific amount of energy an electron in an atom can posses -different electrons, depending on their energies, are found in certain areas |
|
|
|
Orbital |
Region of space occupied by an electron in a particular energy level |
|
|
|
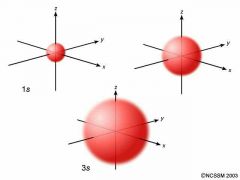
S- orbitals |
|
|
|
|
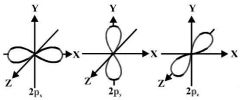
P- orbitals |
|
|
|
|
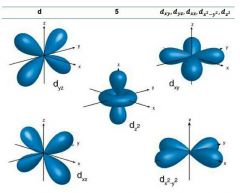
D-orbitals |
|
|
|
|
F-orbitals |

|
|
|
|
s, p, d, f refer to |
The 4 different types of orbitals |
|
|
|
Shell |
Set of all orbitals having the same n-value |
3rd consists of 3s, 3p and 3d orbitals |
|
|
Subshell |
Set of orbitals of the same type |
2nd shell: 1 orbital of the 2s subshell 3 orbitals of the 2p subshell |
|
|
Name the 3 rules electrons have to follow for electron configuration |
1. Aufbau principle 2. Hund's rule 3. Pauli exclusion principle |
|
|
|
Aufbau principle |
Electrons added to the lowest energy level possible |
|
|
|
Hund's rule |
Electrons will occupy orbitals of each subshell singly before filling orbitals in pairs. |
|
|
|
Pauli exclusion principle |
A maximum of 2 electrons can be placed in each orbital and must spin in opposite directions. directions. . |
|
|
|
Order of orbitals for polyelectronic atoms |
1s, 2s, 2p, 3s, 3p, 4s, (3d), 4p, 5s, (4d), 5p, 6s, (4f), (5d) |
|
|
|
Valence electrons |
Are the electrons outside the core taking part in reactions. |
|
|
|
Exceptions to e configuration up to strontium |
1. Chromium: (Ar) 4s1 3d5 2. Copper: (Ar) 4s1 3d10 |
2 |
|
|
Why are there exceptions (what is the cause)? |
A filled or 1/2 (so 5e) filled d-subshell is especially stable. |
|

