![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
matter |
everything that has mass and takes up space |
|
|
mass |
a measure of the amount of matter in an object |
|
|
atom |
a small particle that makes up all matter
|
|
|
an atom is made up of mostly... |
empty space |
|
|
the parts of an atom is |
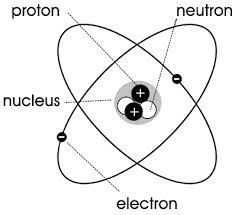
electron, nucleus, neutron, proton |
|
|
definition of weight |
the gravitational force exerted on an object |
|
|
the definition of volume |
the amount of space an object takes up |
|
|
Democritus (460-370 BC) |
the greek philosopher was the first to use the word atom. He believed atoms were solid spheres with no holes. he proposed if u cut paper over and over until you cant anymore you had one atom that can not be divided. |
|
|
the law of conservation of mass (Antoine Lavoisier 1743-1797) |
Lavoisier heated a container of mercury oxide and the powder turned into a silver liquid and gas ( gas was oxygen). the law says mass of the product is the same as the mass of the starting material. |
|
|
the law of definite proportions (J.L. Proust 1799) |
Proust completed experiments that showed any pure compound contains the same elements in the same proportion and that the law applies to every compound. |
|
|
What is the triple beam balance used for? |
to measure mass or amount of a material contained |
|
|
How do you know when the final balance has been reached on a triple beam balance? |
when the grams are back to zero
|
|
|
the definition of chemical reaction
|
a process in which atoms of the starting material rearrange to form a new product with different properties
reactants-------------------product (what you start with)---------(what you end with) |
|
|
the equation iron+oxygen=iron oxide |
Fe+O= FeO |
|
|
the equation for baking soda + vinegar= sodium + acete + water + carbondioxide |
NaHCO + CHCOOH = CHOONa + H20 + CO2 |
|
|
name the 5 principles of the atomic theory (Dalton) |
1. All matter is made up of atoms. 2. Atoms are neither created nor destroyed in chemical reactions. 3. Atoms of different elements combine in whole- number ratios. 4. Each element is made of different kind of atoms. 5. The atoms of different elements have different masses and properties. |
|
|
Dalton's atom |

a simple neutral sphere of indivisible matter that was the same throughout |
|
|
Thomson's atom |
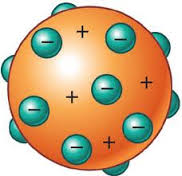
His discovery of electrons showed atoms were not indivisible but contained negative electrons and positive charges. |
|
|
Rutherford's atom |
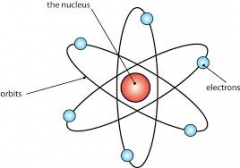
His experiments explained most of the atoms mass is squeezed into the nucleus and the remaining space the electrons moved around the nucleus in any size of a diameter. |
|
|
Bohr' Atom |
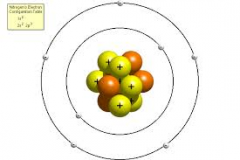
he believed that the electrons had a certain diameter of an orbit |
|
|
Electron cloud atoms |
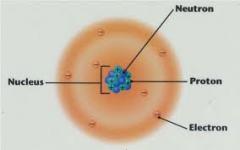
this is the current model in which electrons occupy a space around the nucleus but it is difficult to tell where an electron is |
|
|
explain the scientist Democritus |
he was the first to use the word atom and described the atom as paper not being able to be divided anymore. his model of the atom was a solid sphere. |
|
|
explain the scientist Lavoisier |
he made the law of conservation of mas and the mass of the product is the same as the starting material |
|
|
explain the scientist Dalton |
He invented symbols for elements, the 5 principles in an atomic theory, and measured reactants and products of chemical reactions. |
|
|
explain the scientist Rutherford |
Rutherford did the gold foil experiment and expected to fid out in the experiment that electrons & (+) charges were mixed but the result of the evidence was the (+) charges were mixed evenly because the particles bounced off the foil. |
|
|
explain the scientist Proust |
Proust invented the principle of any pure compound always contains the same elements in the same proportion by mass which came to be known as the law of definite proportions. |
|
|
explain the scientist Thomson |
Thomson was the first to use the name electron. this was found because the cathode ray bent and proposed that there must be positive charges to balance out the negative charges. |
|
|
explain the scientist Chadwick |
In 1932 Chadwick was the first to use the word neutron and proved that there was another undiscovered particle (the neutron) in an atom. |
|
|
explain the scientist Bohr |
Bohr believed electrons can move in circles from a certain diameter around the nucleus. |
|
|
what is a spectral line? |
a single wavelength of light that can be seen when the light from an excited element is passed through the prism |
|
|
explain energy levels |
they are the regions of space that electrons can move around the nucleus |
|
|
what is an element? |
a pure substance made from atoms that all have the same number of protons. |
|
|
what is an atomic number? |
the number of protons in the atom of an element |
|
|
what is a mass number? |
the sum of the number of protons and neutrons the atom has |
|
|
what is an isotope? |
atoms of the same element that contains different numbers of neutrons |
|
|
what is the average atomic mass? |
it is the weighted average mass of the mixture of an element' isotope |
|
|
what is an ion? |
an atom that is no longer neutral because it has gained or lost electrons. Ions form ionic compounds. |

