![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
|
Give 5 steps to evolution of the atmosphere |
1) protosolar disc was the source. 2) inner planets were rocky. Outer planets were gassy. 3) moon forming impact destroyed he early atmosphere. 4) outgassing of methane, ammonia, hydrogen, co2 and CO. 5) ammonia was converted to nitrogen by UV photons. |
|
|
Two key processes for an oxygen rich atmosphere |
1) loss of hydrogen- subduction of hydrated minerals, outgassing of volcanoes which occurred because hydrogen is light. 2) photosynthesis - a lot of oxygen is needed to build up a sufficient amount because oxygen reacts with everything in the atmosphere. |
|
|
Why is the atmosphere rich in argon? |
Radiogenic decay of K |
|
|
Where did water on Earth come from? |
High D/H ratio on Earth is derived from comets and comets. Mass dependant isotopic fractionation caused this difference. |
|
|
Volume mixing ratio |
The volume of air occupied by a gas and is proportional to the amount of molecules in a given volume. |
|
|
Mass mixing ratio |
The fraction of mass of an air parcel contributed by a gas and depends on molecular mass. |
|
|
How many pascals and bars in one atmosphere? |
101325 Pa 1013.25 hPa 1.01325 bars |
|
|
3 ingredients for an atmosphere |
1) mass big enough to prevent solar winds blowing away gas. 2) angular energy source (sun) to keep it warm and in gas form. 3) substance. |
|
|
Describe atmospheric circulation |
Northeast and southeast trade winds. Low pressure at equator. High pressure at horse latitudes and descending air. Westerlies between 30 and 60 degrees. Low pressure rising air at 60 degrees. Polar easterlies between 60 and 90 degrees. High pressure descending air at poles. |
|
|
What is an ideal gas and what are the two equations |
The molecules do not interact with each other. PV = nRT PV = NkT (k = Boltzmann constant) |
|
|
Density of a gas equation |
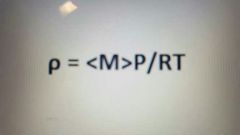
|
|
|
Hydrostatic equation |

|
|
|
Pressure and altitude equation |

|
|
|
Pressure scale height (define and write) |
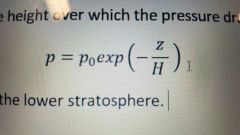
The height over which the pressure drops by 1/e. H = 7 km for the troposphere. |
|
|
Define and derive the equation for thr adiabatic lapse rate |
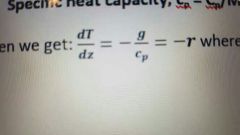
The rate of cooling of air when no heat leves or enters the system. |
|
|
Anabatic wind |
Caused by high altitude air contacting warm surfaces (mountains). Travel uplsope and the heating decreases density. |
|
|
Katabatic wind |
Caused by high altitude winds contacting cold surfaces E.g. Antarctic ice sheet. Cooling increases density and air moves down slope. |
|
|
Dry lapse rate |
10 K/km |
|
|
Saturated lapse rate |
6 K/km |
|
|
What is Archimedes' principle and what is the equation? |

The buoyant force is equal to the weight of a fluid. |
|
|
What is the effective temperature and what is the equation? |
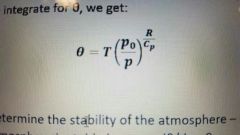
The temperature a parcel of air would have if it moved adiabatically to a standard pressure. |
|
|
Atmospheric inversions |
Horizontal layers where temperature increases with altitude, trapping fog, haze and pollutants e.g. starosphete and thermosphere. |
|
|
Describe the relationship between half life and specific activity |
Radioactive material with long half lives have low specific gravity. |

