![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
142 Cards in this Set
- Front
- Back
|
What is better for cancer: poly or monotherapy?
|
Polytherapy
|
|
|
What are the principles for polypharmacy?
|
Use drugs that have demonstrated activity against the particular cancer being treated
Minimize overlapping host toxicities Maximize mechanistic diversity |
|
|
What is the definition of adjuvant therapy?
|
Chemotherapy after sugery/radiation to eliminate residual disease
|
|
|
What are the risks inherent to adjuvant therapy?
|
You could be blasting someone with chemo who's already cured
|
|
|
What is the definition of neoadjuvant therapy?
|
Chemotherapy before surgery/radiation, to shrink the tumor down to a size that can be surgically removed or treated with radiation
|
|
|
What is the definition of radiosensitization?
|
Chemotherapy to make tumor cells more sensitive to radiation
|
|
|
What are the grades of toxicity severity?
|
1: mild
2: moderate 3: severe - require hospital 4: life-threatening |
|
|
What is the definition of the maximum tolerated dose?
|
The highest drug dose that doesn't cause unacceptable (grade 3-4) toxicity
|
|
|
What is the definition of dose-limiting toxicity?
|
Toxicity that dictates when a drug treatment should cease escalation/be reduced
|
|
|
How are chemotherapy drugs normally dosed?
|
Based on body surface area (rather than mass): mg/m^2
|
|
|
How do you calculate body surface area?
|

With a monogram!
|
|
|
What are the different descriptors of responses to chemotherapy?
|
Cure: 5+ years with no disease
Complete response/remission: no detectable disease < 5 years Partial response/remission: at least 50% shrinkage Stable disease: tumor stays same size Progressive disease: tumor increases in size during treatment |
|
|
What are the major mechanistic categories of anticancer drugs?
|
Direct DNA damaging agents
Antimetabolites Inhibitors of chromosomal structure/organization Signalling pathway modulators |
|
|
What is the mechanism of the direct DNA damaging agents?
|
Chemicals that cause DNA strand breaks or covalent modifications
|
|
|
What is the mechanism of the antimetabolites?
|
Analogs of purines/pyrimidines and their nucleosides that inhibit metabolism of normal counterparts,including substituting for them within RNA and DNA
|
|
|
What is the mechanism of the inhibitors of chromosomal structure/organization?
|
Microtubule antagonists
Topoisomerase inhibitors |
|
|
What are some of the mechanisms used by signalling pathway modulators?
|
Tyrosine kinase inhibitors
Angiogenesis inhibitors Immune response induction Steroid hormone response modifiers |
|
|
What are the different classes of drugs that can cause direct DNA damage?
|
Alkylating crosslinkers: nitrogen mustards, nitrosureas
Non-Alkylating crosslinkers: platinum drugs |
|
|
What are some of the drugs that are alkylating crosslinkers?
|
Mechlorethamine
Cyclophosphamide Carmustine Lomustine |
|
|
What kinds of drugs are derived from nitrogen mustards? What class/general mechanism do they belong to?
|
Mechlorethamine
Cyclophosphamide Class: Aklylating crosslinkers Mechanism: Direct DNA damage |
|
|
What kinds of drugs are derived from nitrosoureas? What class/general mechanism do they belong to?
|
Carmustine
Lomustine Class: alkylating crosslinkers Mechanism: direct DNA damage |
|
|
What's the mechanism of the alkylating cross linkers?
|
DNA-DNA interstrand crosslinking
|
|
|
What types of alkylating crosslinkers require metabolic activtion?
|
Cyclophosphamide
Lomustine |
|
|
What is the mechanism of resistance to the alkylating crosslinkers?
|
Increased inactivation by glutathione, other thiol-containing proteins
Increased DNA repair |
|
|
What's the route of elimination for mechlorethamine?
|
Decomposition
|
|
|
What's the route of elimination for cyclophosphamide?
|
Hepatic metabolism
Renal excretion |
|
|
What's the route of elimination for carmustine?
|
Hepatic metabolism and decomposition
|
|
|
What's the route of elimination for lomustine?
|
Renal excretion
|
|
|
What side effects are shared by all of the alkylating crosslinkers?
|
Myelosuppression:
1-2 week nadir in the mustard drugs 4-6 week nadir in the nitrosourea drugs Severe nausea and vomiting |
|
|
What side effect (from among the alkylating crosslinkers) is unique to cyclophosphamide?
|
Cyclophosphamide (also for ifosfamide)
|
|
|
What drug are you able to give to people to protect against hemorrhagig cystitis for people on cyclophosphamide?
|
MESNA
Also can give this in ifosphamide |
|
|
Under what situations do you have to adjust the dose for:
Mechlorethamine? Cyclophosphamide? Carmustine? Lomustine? |
Mechlorethamine: none
Cyclophosphamide: renal dysfunction Carmustine: none Lomustine: renal dysfunction |
|
|
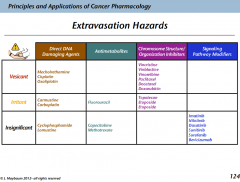
Which of the alkylating crosslinkers have a risk of extravasation?
|
Mechlorethamine: vesicant
Carmustine; irritant |
|
|
What are the non-alkylating crosslinkers?
|
Cisplatin
Carboplatin Oxaliplatin |
|
|
What types of a drugs are cisplatin, carboplatin, and oxaliplatin?
|
Non-alkylating crosslinkers
|
|
|
What's the biochemical activity of the non-alkylating crosslinkers?
|
DNA-DNA interstrand crosslinking
|
|
|
What's the resistance mechanism to the non-alkylating crosslinkers?
|
Increased inactivation by glutathione and other thiol containing proteins
Increased DNA repair |
|
|
What's the elimination of the non-alkylating crosslinkers?
|
Renal excretion
|
|
|
What are the common toxicities of cisplatin?
|
RENAL TOXICITY!: DLT, cumulative
Neurotoxicity Ototoxicity Severe nausea, vomiting |
|
|
What is the dose-limiting toxicity of cisplatin?
|
Renal toxicity
|
|
|
What is the dose-limiting toxicity of carboplatin?
|
Myelo-suppression
|
|
|
What is the dose-limiting toxicity of oxaliplatin?
|
Neutrotoxicity
It's also cumulative! |
|
|
What are the toxicities of carboplatin?
|
Renal toxicity
Myelosuppression (DLT!) Severe nausea, vomiting |
|
|
What are the toxicities of oxaliplatin?
|
Renal toxicity
Myelosuppression Neurotoxicity: DLT, CUMULATIVE! |
|
|
When do you have to dose adjust the non-alkylating crosslinkers?
|
Renal dysfunction
|
|
|
What non-alkylating cross linker requires IV hydration and diuresis?
|
Cisplatin
Because of the renal toxicity |
|
|
What are the extravasation hazard of the non-alkylating crosslinkers?
|
Cisplatin: vesicant
Carboplatin: irritant Oxaliplatin: vesicant |
|
|
What drugs are thymidine nucleotide antagonists?
|
Fluorouracil
Capecitabine Pemetrexed Methotrexate |
|
|
What is the mechanism of fluorouracil, capecitabine, pemetrexed, and methotrexate?
|
Tymidine nucleotide antagonists
|
|
|
What is the mechanism of fluorouracil?
|
1. Metabolism to FdUMP
2. FtUMP/TS/folate covalent complex formed INHIBITION OF THYMIDYLATE SYNTHASE |
|
|
What is the effect of inhibiting thymidylate synthase?
|
Stalled replication
Uracil-DNA incorporation DNA strand breaks |
|
|
What is the biochemical action of capecitabine?
|
1. Metabolism to 5FU
2. Metabolism to FdUMP 3. Formation of FdUMP/TS/folate covalent complex THYMIDYLATE SYNTHASE INHIBITION |
|
|
Which of the thymidine nucleotide antagonists are uracil analogs? Folate analogs?
|
Uracil analogs: fluorouracil, capecitabine
Folate: pemetrexed, methotrexate |
|
|
What is the mechanism of pemetrexed?
|
Tight binding to TS at the folate site
TS inhibition |
|
|
What is the mechanism of methotrexate?
|
1. Tight binding to DHFR at the folate site
2. Depletion of the active (reduced) folates TS inhibition |
|
|
What is necessary for the activity of thymidylate synthase?
|
Folate in the folate binding site
Nucleotide |
|
|
What is the relationship between fluorouracil and capecitabine?
|
Capecitabine is metabolized into 5FU
|
|
|
What is the mechanism of resistance to 5FU and capecitabine?
|
Increased thymidylate synthase
Reduced folate cofactor |
|
|
What are the mechanisms of resistance to pemetrexed?
|
Increased TS
Decreased drug uptake Decreased drug retention |
|
|
What are the mechanisms of resistance to methotrexate?
|
Increased DHFR
Decreased drug uptake, retention Decreased conjugation to glutamate groups (needs to be conjugated to stay inside the cell) |
|
|
What's the route of elimination for:
5FU? Capecitabine? Pemetrexed? Fethotrexate? |
5FU: hepatic metabolism
Capecitabine: hepatic metabolism Pemetrexed: renal excretion Fethotrexate: renal excretion The uracil drugs are the same elimination The folate analogs are the same elimination |
|
|
What's the relationship between thymidylate synthase and folates?
|
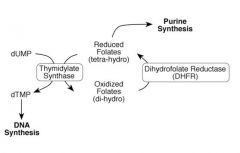
|
|
|
What reaction is performed by thymidylate synthase?
|
dUMP --> dTMP
|
|
|
What kind of cofactor is required for the activity of thymidylate synthase?
|
Reduced folate (THF)
they donate a carbon |
|
|
What enzyme regenerates folates for dTMP synthesis?
|
Dihydrofolate reductase
|
|
|
What agent can you give along with 5FU to incrase toxicity?
|
Leucovorin
|
|
|
What's the mechanism for increasing 5FU toxicity with leucovorin?
|
It allows folate depleted tumor cells to keep their TS running, which increases toxicity
|
|
|
What are the different mechanisms of inhibiting choromsomal structure/organization in chemotherapy?
|
Microtubule antagonists
Topoisomerase inhibitors |
|
|
What are the different agents that work on microtubules?
|
Vinca alkaloids: microtubule destabilization
Taxane: microtubule stabilization |
|
|
What's the mechanism of the TOPO1 inhibitors?
|
After the topo1 makes a snip in the DNA, it can't bind on the other side of the snip, meaning that the DNA can't be annealed back together again
|
|
|
How do to the topoisomerase II inhibitors work?
|
They stabilize TOPOII when it's on the DNA, ultimately leading to protein disruption and a DS break
|
|
|
What is the mechanism of the vinca alkaloids?
|
Microtubule destabilizers
|
|
|
What are the different vinca alkaloid derivatives used in chemotherapy?
|
Vincristine
Vinblastine Vinorelbine |
|
|
What is the mechanism of resistance to the vinca alkaloids?
|
MDR mediated by P-glycoprotein
Mutations int he tubulin protein that decrease drug affinity |
|
|
What's the route of elimination for the vinca alkaloids?
|
Hepatic metabolism
|
|
|
What is the DLT of vincristine?
|
Neurotoxicity
|
|
|
What is the DLT of vinblastine and vinorelbine?
|
Myelosuppression
|
|
|
What are the toxicities of vinoblastine and vinorelbine?
|
Myelosuppression (DLT)
Neurotoxicity |
|
|
When do you have to dose adjust the vinca alkaloids?
|
When there's hepatic dysfunction
|
|
|
What is the extravasation hazard for the vinca alkaloids?
|
Vesicants
|
|
|
What is the mechanism of the taxanes?
|
Microtubule stabilizers
|
|
|
What are the drugs derived from taxanes used in chemotherapy?
|
Paclitaxel
Docetaxel |
|
|
What's the mechanism of resistance to the taxanes?
|
MDR mediated by PgP
Mutations in tubulin that decrease drug affinity |
|
|
What's the route of elimination of the taxanes?
|
Hepatic metabolism
|
|
|
What are the toxicities of the taxanes?
|
Myelosuppression
Neurotoxicity Hypersensitivity: common and severe Skin toxicity |
|
|
What do you have to do because of the hypersensitivity reactions to the taxanes?
|
Premedicate to prevent it
|
|
|
When do you have to dose adjust the taxanes?
|
Hepatic dysfunction
|
|
|
What's the extravasation risk with the taxanes?
|
Vesicants
|
|
|
What's the DLT of the taxanes?
|
Myelosuppression
|
|
|
What is leucovorin?
|
A precursor to folate
Increases the toxicity of 5-FU Rescues cells from MTX |
|
|
How does leucovorin rescue cells from MTX toxicity?
|
MTX is inhibiting their DHFR, so they need a source of folate.
In tumor cells, there aren't enough transporters to import the leucovorin, but there are in regular cells. The folate from leucovorin is enough to save those cells |
|
|
What drugs inhibit topoisomerase 1?
|
The camptothecins:
Topotecan Irinotecan |
|
|
What is the mechanism of the camptothecins: topotecan and irinotecan?
|
Inhibition of TOPOI
|
|
|
What is the way that resistance is developed to the camptothecins?
|
MDR mediated by PgPs
Mutations in TOPOI that decrease drug affinity Decreased expression of TOPOI |
|
|
What are the mechanisms of resistance to irinitecan?
|
MDR mediated by PgPs
Mutations in TOPOI that decrease drug affinity Decreased expression of TOPOI Decreased metabolic converstion to SN-39 |
|
|
What is a requirement for the activityof irinotecan?
|
Metabolic converstion to SN-38
|
|
|
What is the route of elimination of topotecan?
|
Renal excretion
|
|
|
What is the route of elimination of irinitecan?
|
Biliary excretion
|
|
|
What are the toxicities of the TOPOI inhibitors?
|
Myelosuppression
GI mucositis Nausea and vomiting (rare) |
|
|
What is the DLT of topotecan?
|
Myelosuppression
|
|
|
What is the DLT of irinotecan?
|
GI mucositis
|
|
|
When do you have to dose adjust topotecan?
|
Renal dysfunction
|
|
|
When do you have to dose adjust irinitecan?
|
Hepatic dysfunction
|
|
|
What are the extravasation hazards of the TOPOI inhibitors?
|
They're only irritants
|
|
|
What are the TOPOII inhibitors?
|
Doxorubicin
Etoposide |
|
|
What is the mechanism of Doxorubicin and Etoposide?
|
TOPOII inhibitors
|
|
|
What is the mechanism of Doxorubicin?
|
TOPOII inhibition
Oxygen free radical formation-->DNA strand breaks |
|
|
What is the mechanism for etoposide?
|
TOPOII inhibition
|
|
|
What is the mechanism of resistance to doxorubicin?
|
MDR mediated by PgP
Mutations in TOPOII that decrease affinity Decreased expression of TOPOII Incrased inactivation by glutathione |
|
|
What is the mechanism of resistance to etoposide?
|
MDR mediated by PgP
Mutations in TOPOII that decrease affinity Decreased expression of TOPOII |
|
|
What is the route of elimination for doxorubicin?
|
Biliary excretion
|
|
|
What is the route of elimination for Etoposide/
|
Renal excretion
Hepatic metabolism |
|
|
What are the toxicities of Doxorubicin?
|
Myelosuppression
Cardiotoxicity GI mucositis Nausea and vomiting |
|
|
What are the toxicities of Etoposide?
|
Myelosuppression
GI mucositis Nausea and vomiting |
|
|
In what conditions do you need to adjust the dose of the TOPOII inhibitors?
|
Renal or hepatic dysfunction
|
|
|
What are the extravasation hazards for doxorubicin?
|
Vesicant
|
|
|
What are the extravasation hazards for etoposide?
|
Irritant
|
|
|
What are different ways that signalling pathways can be altered in anticancer drugs?
|
Tyrosine kinase inhibitors
Angiogenesis inhibitors Immune response induction Steroid hormone response modifiers |
|
|
What is most common way to alter signalling pathways with drugs?
|
Antibodies!
|
|
|
What particular tyrosine kinase causes problems in cancer, commonly? What causes it?
|
BCR-ABL
It comes from the Philly chromosome |
|
|
What are the drugs that inhibit the TK Bcr-Abl?
|
Imatinnib
Nilotinib Dasatinib |
|
|
What is the action of imatinib, nilotinib, and dasatinib?
|
Inhibition of Bcr-Abl, a tyrosine kinase
|
|
|
What additional enzyme does dasatinib inhibit?
|
Src kinase
|
|
|
What is the mechanism of resistance to the tyrosine kinase inhibitors?
|
Bcr-Abl mutations
Increased Bcr-Abl expression |
|
|
What is the route of elimination for the tyrosine kinase inhibitors?
|
Hepatic metabolism
|
|
|
What are the toxicities for the tyrosine kinase inhibitors?
|
Myelosuppression
CNS toxicity Skin toxicity Lung toxicity CV toxicity |
|
|
In what situations do you need to dose adjust the tyrosine kinase inhibitors?
|
Hepatic dyrfunction
|
|
|
What are the different angiogenesis inhibitors?
|
Bevacizumab
Sunitinib Sorafenib |
|
|
What is the class of Bevacizumab, Sunitinib, and Sorafenib?
|
Angiogenesis inhibitors
|
|
|
What is the mechanism of bevacizumab?
|
Binding to free VEGF-->inhibition of angiogenesis signalling
|
|
|
What is the mechanism of Sunitinib and Sorafenib?
|
Binding to VEGF receptor-->inhibiting angiogenic signalling
Inhibition of other receptor kinases |
|
|
What's the mechanism of resistance to:
Bevacizumab? Sunitinib? Sorafenib? |
Bevacizumab: unknown
Sunitinib: unknown Sorafenib: expression of targeted kinases |
|
|
What's the route of elimination for bevacizumab?
|
Gradual degradation
|
|
|
What's the route of elimination for sunitinib?
|
Hepatic metabolism
|
|
|
What's the route of elimination for sorafenib?
|
Hepatic metabolism
|
|
|
What are the toxicities to the angiogenesis inhibitors?
|
CV toxicitiy (HTN, thromboembolism)
CNS toxicity (headaches, fatigue) Hemorrhage Myelosuppression |
|
|
When should you dose adjust the angiogenesis inhibitors?
|
Sunitinib: hepatic dysfunction
Sorafenib: hepatic dysfunction |
|
|
What signalling pathway do the angiogenesis inhibitors act against?
|
VEGF signalling
|
|
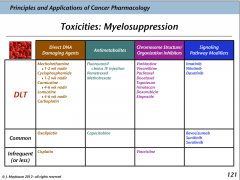
Myelosuppression
|
Killing of the bone marrow
|
|
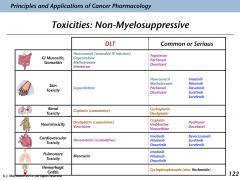
Nonmyelosuppressive toxicities
|
Ways that toxicities occur
|
|
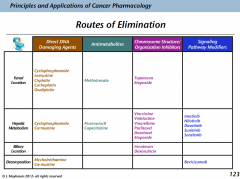
Routes of elimination
|
Ways that elimination occurs
|
|
You can bleed out!
|
Not good.
|

