![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
110 Cards in this Set
- Front
- Back
|
water |
cells rely on this as a diffusion medium for the distribution of gases, nutrients, and wastes. -when content is low: proteins denature, enzymes cease functioning, and cells die. |
|
|
extracellular fluid (ECF) |
the interstitial fluid, plasma, and other bodily fluids. -minor components = lymph, CSF, synovial fluid, serous fluids (pleural, pericardial, peritoneal), aqueous humor, perilymph, and endolymph. -not much variation in composition. |
|
|
intracellular fluid (ICF) |
the cytosol (fluid inside a cell) -has the greatest variation as a result of the differences in the content of fat vs muscle. |
|
|
concentrations of K and Ca ions in the ECF become too high |
cardiac arrhythmias develop and death can result. |
|
|
power of hydrogen (pH) |
-if this is too high, can break chemical bonds and change the shapes of complex molecules, disrupt plasma membranes, and impair tissue functions. |
|
|
Fluid balance |
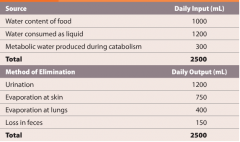
-amount of water gained each day is equal to the amount lost to the environment. -maintenance involves regulating the content and distribution of body water in the ECF and ICF. -cells transport ions and create concentration gradients that are then eliminated by osmosis. |
|
|
Electrolytes |
-ions released when inorganic compounds dissociate. -can conduct electrical current in solution. -gained from food and drinks. |
|
|
electrolyte balance |
-gains and losses of electrolytes are equal. -involves balancing the rates of absorption across the GI tract with rates of loss by the kidneys (and sweat). -important because: total electrolyte conc. directly affect water balance and the conc. of individual electrolytes can affect cell function. |
|
|
acid-base balance |
-production of hydrogen ions in your body is precisely off-set by their loss. -pH is maintained in normal limits (preventing a decrease in the pH is the primary problem) -secretion of H ions takes place primarily in the DCT + collecting ducts of kidneys, along with generating buffers. -lungs eliminate CO2. |
|
|
Body composition (Female vs. Male) |
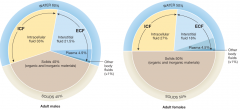
|
|
|
clinical surveillance of water comp. |
-safe to estimate that 2/3 of total body water is in ICF. -portions of the ECF (water in bone, in many dense connective tissues, and in minor components) are relatively isolated so fluid exchange occurs more slowly here than that between plasma and other interstitial fluids. -attention usually focused on rapid fluid and solute movements that accompany the administration of blood, plasma, or saline solutions to counteract blood loss or dehydration. |
|
|
exchange among the subdivisions of the ECF |
-occurs primarily across the endothelial lining of capillaries. -may also travel from the interstitial spaces to plasma through lymphatic vessels and drain into the venous system. |
|
|
cations and anions in fluid compartments |
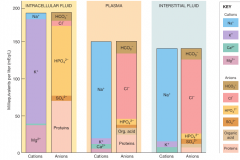
|
|
|
plasma membranes are selectively permeable: |
-ions can enter or leave the cell only by specific membrane channels. |
|
|
osmotic concentrations of ICF and ECF |
-identical, despite the differences in the concentration specific substances. -osmosis eliminates minor differences in concentration almost at once, because most plasma membranes are freely permeable to water. (except along the ascending limb of nephron loop, DCT, and collecting duct) |
|
|
Four Basic Principles of Fluid and Electrolyte Balance: |
1. all the homeostatic mechanisms that monitor and adjust the composition of body fluids respond to changes in the ECF, not in the ICF. 2. no receptors directly monitor fluid or electrolyte balance. 3. cells cannot move water molecules by active transport. 4. the body's content of water or electrolytes will increase if dietary gains exceed losses to the environment, and will decrease if losses exceed gains. |
|
|
receptors monitoring the composition of ECF |
-detect significant changes in their composition or volume and trigger appropriate neural and endocrine responses. -change in one ECF component spreads rapidly because it is not isolated via plasma membranes. |
|
|
three hormones that mediate physiological adjustments to fluid/electrolyte balance: |
1. Antidiuretic hormone (ADH) 2. aldosterone 3. natriuretic peptides (ANP & BNP) |
|
|
osmoreceptors |
-special cells in the hypothalamus that monitor the osmotic concentration of ECF. -sensitive to subtle changes. -includes neurons that secrete ADH near fenestrated capillaries in the posterior lobe of the pituitary gland. (higher osmotic concentration = more ADH release) |
|
|
increased release of ADH: |
1. stimulates water conservation by the kidneys, reducing urinary water loss and concentrating the urine. 2. stimulates the hypothalamic thirst center. |
|
|
aldosterone |
-secreted by the adrenal cortex (synth in the liver). -major role in determining the rate of Na absorption and subsequent K loss along the DCT and collecting system. (more = Na conservation, which also means water retention..."water follows salt") -increase sensitivity of salt receptors on tongue to increase uptake. -released in response to increased K or decreased Na levels in the blood reaching the adrenal cortex or response to activated RAAS. |
|
|
rennin release in response to: |
1. decrease in plasma blood volume/pressure at Juxt complex. 2. decrease in the osmotic conc. of tubular fluid @ DCT. 3. decrease Na or increase K conc. in renal circulation. |
|
|
natriuretic peptides (ANP and BNP) |
-released by cardiac muscle cells in response to abnormal stretching of the heart walls caused by increased blood pressure or volume. -reduce thirst and block the release of ADH and aldosterone. |
|
|
diuresis |
fluid loss by the kidneys with release of natriuretic peptides. -decreases blood pressure and plasma volume, eliminating heart wall overstretching. |
|
|
water vs. electrolyte balance
|
-considered separately even though closely related. -regulatory mechanisms are quite different. -distinction is necessary clinically when there are problems with fluid balance and electrolyte balance that must be identified. |
|
|
hydrostatic pressure |
-forces water out of blood plasma and into interstitial space. -some of that water may be reabsorbed in the distal portion of the capillary bed and the rest enters lymphatic vessels for transport to the venous circulation. |
|
|
fluid movement among the minor components of the ECF |
-is continuous. |
|
|
flow rate of water moving back and forth across the mesothelial surfaces that line the cavities: |
-significant -but the actual volume present at any one time is very small. |
|
|
Na / water ratio |
provides useful clues to the state of water balance. |
|
|
interstitial fluid and fluid compartments contain approx: |
80% of the ECF volume and plasma contains the other 20%. |
|
|
edema |
any factoring altering the net hydrostatic pressure or the net colloid osmotic pressure will alter the distribution of fluid within the ECF resulting in this. |
|
|
causes of edema: |
-increase blood pressure -decreased blood colloid osmotic pressure (advanced starvation, plasma protein decrease) -damaged capillary wall -constriction of regional venous circulation -blockage of lymphatic drainage |
|
|
fluid gains and losses |
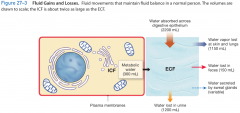
|
|
|
metabolic generation of water |
-production of water within cells, primarily as a result of oxidative phosphorylation in mitochondria. -diet in US: 46% carbs, 40%lipid, 14% protein = 300 mL of water per day (12% daily requirement) |
|
|
Fluid Shift |
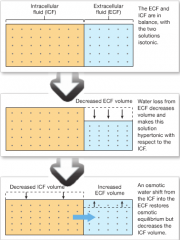
-rapid water movement between the ECF and ICF in response to an osmotic gradient. -occur quickly in response to changes in osmotic concentration of the ECF and reach equilibrium within minutes to hours. |
|
|
dehydration |
"water depletion" -develops when water losses outpace water gains. -body fluids become more concentrated and sodium ion conc. become abnormally high (hypernatremia) -if this imbalance remains unchecked, the loss of water will produce severe thirst, dryness, and wrinkling of the skin. -shock may develop if severe enough. |
|
|
First week of dieting |
-may seem unproductive because your body is conserving water and catabolizing lipids. |
|
|
causes of hyperhydration |
-drinking too much water or infusion of a hypotonic sol. -inability to eliminate excess water in urine (chronic renal failure, heart failure, cirrhosis, etc) -endocrine disorders (too much ADH prod.) |
|
|
first signs of hyperhydration |
-effects on CNS. -individual acts drunk (water intox) -can be deadly. |
|
|
equivalent (eq) |
electrolytes in the body fluids are measured in terms of this. -amount of positive or negative ion that supplies one mole of electrical charge. |
|
|
two general rules concerning sodium and potassium balance worth noting: |
1. the most common problems with electrolyte balance are caused by an imbalance between gains and losses of sodium ions. 2. problems with K balance are less common but significantly MORE dangerous than problems related with Na. |
|
|
Homeostatic regulation of increased [Na] in ECF |
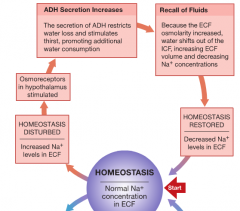
|
|
|
Homeostatic regulation of decreased [Na] in ECF |
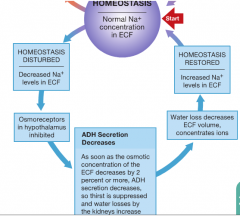
|
|
|
Fluid Volume regulation and [Na] in Body fluids (increasing blood pressure and volume) |
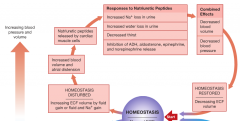
|
|
|
Fluid Volume regulation and [Na] in Body fluids (decreasing blood pressure and volume) |
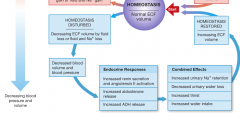
|
|
|
baroreceptors at the carotid sinus, aortic sinus, and the right atrium. |
-homeostatic mechanisms can monitor ECF volume indirectly by monitoring blood pressure via these receptors. |
|
|
rate of tubular secretion of K varies based on three factors: |
1. changes in the [K] of ECF. 2. changes in pH. 3. aldosterone levels |
|
|
major factors involved in disturbances of potassium balance |

|
|
|
volume depletion |
occurs when athletes sweat lose much more water than they do sodium. (increase [Na] in ECF) -kidney function is impaired because this occurs at the same time that blood is being shunted away from the kidneys. -wastes accumulate in the blood. |
|
|
calcium |
-the most abundant mineral in the body. -99% is in the skeleton -also play role in muscular and neural activities, blood clotting, as cofactors for enzymatic reactions, and as second messengers.
|
|
|
increase [Ca] |
-parathyroid hormone -calcitriol |
|
|
decrease [Ca] |
calcitonin. |
|
|
magnesium |
-required as a cofactor for several important enzymatic reactions, including phosphorylation of glucose within cells and the use of ATP by contracting muscle fibers. |
|
|
phosphate ions |
-required for bone mineralization -inside cells, required for the formation of high-energy compounds, the activation of enzymes, and the synthesis of nucleic acids. |
|
|
chloride ions |
-most abundant anions in the ECF.
|
|
|
Electrolyte balance in Adults |
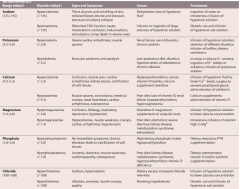
|
|
|
acid-base terminology |

|
|
|
carbonic acid |
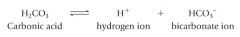
-a weak acid. -at the normal pH of ECF, and equilibrium exists: |
|
|
acidosis |
-acidemia that exists when the pH of the blood falls below 7.35. |
|
|
alkolosis |
-alkemia that exists when the blood pH rises about 7.45. |
|
|
severe acidosis |
-effects primary the CV and nervous systems. 1. CNS function deteriorates and the person can become comatose. 2. cardiac contractions grow weak and irregular, and signs/symptoms of heart failure develop. 3. peripheral vasodilation produces a dramatic decrease in blood pressure, potentially producing circulatory collapse. |
|
|
Three classes of Acids that can THREATEN pH balance |

|
|
|
carbonic anhydrase is found... |
in the cytoplasm of RBCs, liver, and kidney cells, parietal cells of the stomach, etc. |
|
|
P(CO2) is inversely related to the pH |
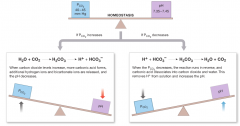
|
|
|
Buffer Systems in Body Fluids |
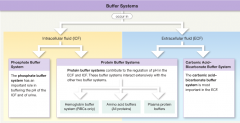
|
|
|
buffers |
-dissolved compounds that stabilize the pH of a solution by adding or removing H ions. -include weak acid and its conjugate base. |
|
|
Phosphate Buffer System |
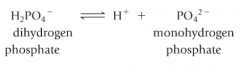
-plays a supporting role in ECF pH regulation -very important in regulating ICF pH and that of urine. |
|
|
protein buffer systems |
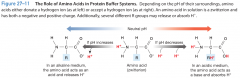
depend on the ability of amino acids to respond to pH changes by accepting or releasing protons. (amphoteric) |
|
|
plasma proteins |
-contribute to the buffering capabilities of the blood.
|
|
|
interstitial fluid and pH regulation |
-contains extracellular protein fibers and dissolved amino acids that assist in this. |
|
|
ICF of active cells |
-structural or other proteins provide an extensive buffering capability. -buffering prevents destructive changes in pH when cellular metabolism produces organic acids such as lactic acid or pyruvic acid. -acid from the ECF can come here to get buffered. |
|
|
Hb Buffer System |
-the only Intracellular buffer system that can have an immediate effect on the pH of the ECF. -helps prevent drastic changes in pH when the plasma P(co2) is increasing or decreasing. |
|
|
Carbonic Acid-Bicarbonate Buffer System |
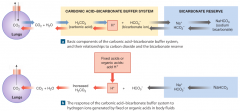
-primary role is to prevent changes in H caused by organic acids and fixed acids in the ECF. -reaction is freely reversible. |
|
|
Important limitation of the carbonic acid-bicarbonate buffer system |
1. it cannot protect the ECF from changes in pH that result from increased or decreased levels of CO2. (its own weak acid) 2. it can function only when the respiratory system and the respiratory control centers are working normally. 3. the ability to buffer acids is limited by the availability of bicarbonate ions. |
|
|
bicarbonate reverse |
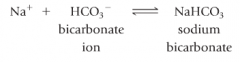
-readily available supply of HCO3- |
|
|
preserving homeostasis through acid-base balance |
-buffers only render the H ions as harmless. (body's supply of buffer is limited)` -captured H ions must ultimately be either permanently tied up in water molecules, through the removal of carbon dioxide by the lungs, or removed from the body fluids, through secretion by the kidneys. |
|
|
respiratory compensation |
-change in the respiratory rate that helps stabilize the pH of ECF. -takes place whenever the body pH strays out of normal limits. -respiratory activity has a direct effect on the carbonic acid-bicarbonate buffer system. |
|
|
chemoreceptors of the carotid and aortic bodies |
-these are sensitive to the Pco2 if circulating blood. -a rise in Pco2 stimulates these receptors leading to an increase in the respiratory rate. |
|
|
renal compensation |
-change in the rates of H+ and HCO3- secretion or reabsorption by the kidneys in response to changes in plasma pH. |
|
|
the ability to eliminate a large number of hydrogen ions in a normal volume of urine |
-depends on the presence of buffers in the urine. -secretion of H+ can only continue until the pH of the tubular fluid reaches 4-4.5 (the point at which H+ concentration gradient is too high and they leak out as fast as they are pumped in) |
|
|
if the tubular fluid lacked buffer |
-the kidneys would secrete less that 1% of the acid it produced each day before the pH reached its limit. -buffers keep the pH high enough for H+ secretion to continue. |
|
|
secretion of H+ ions by the kidney tubules |
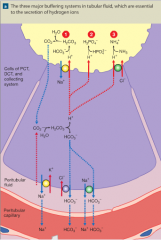
|
|
|
generation of ammonia within the kidney tubules |

In a starving person, tubule cells break down amino acids yielding ammonium ions that are pumped into the tubular fluid, bicarbonates to help buffer ketone bodies in the blood, and carbon chains for catabolism. |
|
|
the response of the kidney tubule to alkalosis |
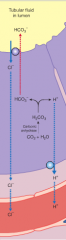
1. the rate of H+ secretion by the kidneys declines. 2. tubule cells do not reclaim the bicarbonates in the tubular fluid 3. the collecting system transports HCO3- into the tubular fluid while releasing a strong acid into the peritubular fluid.
|
|
|
Interactions among the Carbonic Acid-Bicarbonate Buffer System and Compensatory Mechanisms in the Regulation of Plasma pH (acidosis) |
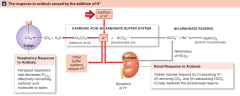
|
|
|
Interactions among the Carbonic Acid-Bicarbonate Buffer System and Compensatory Mechanisms in the Regulation of Plasma pH (alkalosis) |
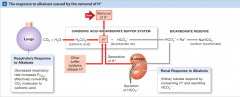
|
|
|
More serious and prolonged disturbances of acid-base balance can result under the following circumstances: |
-any disorder affecting circulating buffers, respiratory performance, or renal function. (emphysema and renal failure) -cardiovascular conditions (heart failure or hypertension cause fluid shifts and change GFR) -conditions affecting the CNS (affect respiratory and cardiovascular reflexes that are essential to pH regulation) |
|
|
respiratory acid-base disorders |
-result from a mismatch between carbon dioxide generation in peripheral tissues and carbon dioxide excretion by the lungs -carbon dioxide levels in ECF are abnormal |
|
|
metabolic acid-base disorders |
-caused by the generation of organic acids or fixed acids or by conditions affecting the concentration of HCO3- in the ECF. |
|
|
respiratory acidosis |
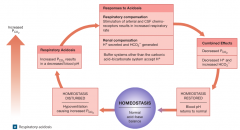
-develops when the respiratory system cannot eliminate all the CO2 generated by the peripheral tissues. (hypercapnia) -most common challenge to acid-base equilibrium. -tissues generate CO2 rapidly, so this occurs within a few minutes of hypoventilation. |
|
|
acute respiratory acidosis |
-an immediate, life-threatening condition in which chemoreceptors do not respond, breathing rate cannot be increased, or the circulatory supply to the lungs is inadequate. -reversal of this is probably the major goal in the resuscitation of cardiac arrest or drowning victims. |
|
|
chronic respiratory acidosis |
-develops when normal respiratory function has been compromised, but the compensatory mechanisms have not failed completely. -chronic hypoventilation of people who have had CNS injuries. -conditions fostering this include: emphysema, congestive heart failure, and pneumonia. (also pneumothorax and respiratory muscle paralysis) |
|
|
respiratory alkalosis |
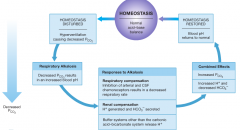
-respiratory activity lowers blood Pco2 to below normal levels. (hypcapnia) -can be produced by hyperventilation which can increase the pH to around 8.0. -this generally corrects itself because the reduction in Pco2 halts the stimulation of chemoreceptors. |
|
|
common causes of hyperventilation |
-physical stresses such as pain, or psychological stresses such as extreme anxiety. symptoms -symptoms that occur include tingling of the hands, lips, and feet, also light-headed. -person may lose consciousness which removes psychological stimulation and the breathing rate decreases.
|
|
|
rebreathe |
this may be required if a person is hyperventilating. -air is breathed out into a bag and the CO2 is recirculated upon the next inhalation. |
|
|
three major causes of metabolic acidosis |
1. production of large number of fixed acids or organic acids. 2. impaired ability to excrete H+ by the kidneys. 3. severe bicarbonate loss. |
|
|
glomerulonephritis |
severe kidney damage that can result in severe metabolic acidosis.
|
|
|
metabolic acidosis from increased acid production/decreased secretion. |
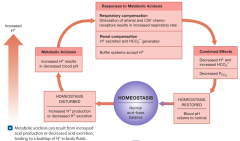
|
|
|
lactic acidosis |
-can develop after strenuous exercise or prolonged tissue hypoxia as active cells rely on anaerobic respiration. |
|
|
ketoacidosis |
-results from the generation of large quantities of ketone bodies during the postabsorptive state of metabolism. -problem in starvation and potentially lethal complication of poorly controlled diabetes mellitus. -peripheral tissues cannot obtain adequate glucose from the bloodstream and they begin metabolizing lipids and ketone bodies. |
|
|
metabolic acidosis from loss of bicarbonate ions |
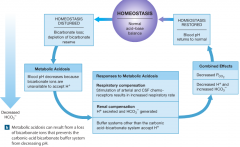
-this buffer system is rendered less effective and acidosis soon develops. -this is most commonly the result of chronic diarrhea. |
|
|
respiratory and metabolic acidosis |
-typically linked because oxygen-starved tissues generate large quantities of lactic acid, and because sustained hypoventilation leads to decreased arterial PO2 |
|
|
metabolic alkalosis |
![-occurs when [HCO3-] are elevated.
-bicarbonate ions then interact with H+ in solution forming H2CO3.
-this is rare.](https://images.cram.com/images/upload-flashcards/63/63/20/9636320_m.png)
-occurs when [HCO3-] are elevated. -bicarbonate ions then interact with H+ in solution forming H2CO3. -this is rare. |
|
|
alkaline tide |
-due to the influx into the ECF of large numbers of bicarbonate ions associated with the secretion of HCl by the gastric mucosa. -temporarily increased the HCO3- concentration in the ECF during meals. |
|
|
vomitting |
may bring about serious metabolic alkalosis because the stomach continues to generate stomach acids to replace those that are lost. |
|
|
treatment of acute metabolic alkalosis |
-administering ammonium chloride -metabolism of ammonium ion liberates a H+ |
|
|
ACID-BASE DISORDERS |
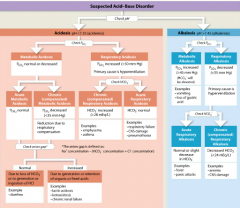
|
|
|
changes in blood chemistry associated with the major classes of ACID-BASE DISORDERS |
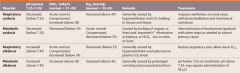
|
|
|
identifying possible causes of the problem and distinguishing compensated from uncompensated conditions |
-steps may include determining the anion gap, plotting blood test results on a numerical graph (nomogram), or using a diagnostic chart. |
|
|
Aging affects several aspects of fluid, electrolyte, and acid-base balance |
-total body water gradually decreases -decrease in GFR and #nephrons -lack of ability to concentrate urine, so more water is lost. -reduction in ADH and aldosterone sensitivity -net loss in body mineral content as muscle mass and skeletal mass decrease. -reduction in vital capacity. -disorders affecting major systems become more common with increasing age. |

