![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
81 Cards in this Set
- Front
- Back
|
Define selectivity |
The ability to kill microbes without harming the host |
|
|
What does an agent that has absolute selectivity do?
|
Targets a structure that we absolutely do not have in our bodies
|
|
|
Are antiviral treatments very selective?
|
No
|
|
|
Are fungi and parasites eukaryotic or prokaryotic? What kind of selectivity will animicrobials to them have?
|
Eukaryotic. It will be relative selective.
|
|
|
What are the two classes of antibiotics? What do they do?
|
Bactericidal (kill bacteria) and bacteriostatic (stop growth)
|
|
|
How do bacteriostatic drug usually work?
|
Binds revesibly with the bacteria and doesn't let it work.
|
|
|
What is the goal of bacteriostatic drugs?
|
You limit the growth enough so that the host can use their immune system to do the rest. This is limited in immunocompromised people.
|
|
|
How does sulfanilamide work? How does it make bacteria static?
|
Inhibits the synthesis of folate. Once the existing folate is used up, the bacteria start plateauing in growth.
|
|
|
How do aminoglycosides get into bacteria?
|
It is cotransported with oxygen in the electron transport chian.
|
|
|
What are the three main targets of antibiotics?
|
1. Cell wall synthesis
2. DNA synthesis 3. Protein synthesis (ribosomes) |
|
|
How do beta-lactams work? What class are they?
|
They bind to transpeptidase preventing protein crosslinkages in the peptidoglygcan wall. It is bacteriocidal.
|
|
|
How do glycopeptide antibiotics work?
|
They bind to the terminal AA of the 4/5 peptide chain and inhibit cell wall synthesis that way.
|
|
|
What are the 4 types of beta-lactam antibiotics?
|
1. Penicillin
2. Cephalosporin 3. Carbapenem 4. Monobactam |
|
|
What type of bacteria can traditional penicillin be used for? Why?
|
Only gram positive because it can't pass through the capsule.
|
|
|
WHat is another drawback to penicillin?
|
It is susceptible to betalactamase.
|
|
|
Where does betalactamase come from?
|
bacteria like staph aureus
|
|
|
What is ampicillin?
|
A variation of penicllin that has an added amino group so it can penetrate gram negative.
|
|
|
What is amoxicillin.
|
A variation of penicllin that can penetrate gram negative and can also be absorbed orally.
|
|
|
What is methicillin (nafcillin, oxacillin)
|
A variation of penicllin that is resistant to beta lactamase, but has lost it's ability to penetrate gram negative.
|
|
|
WHat's so special about cephalosporins?
|
They are resistant to beta lactamase.
|
|
|
How are cephalosporins classified?
|
By generation. These are additional modifications that have been made to increase their effectiveness.
|
|
|
Describe the pros carbapenems.
|
These have the broadest spectrum of all the beta lactams and are resistant to betalactamase.
|
|
|
Describe the drawbacks of carbapenems.
|
They are not absorbed well so can only be given by IV as a last resort
|
|
|
Describe monolactams/
|
They have been modified so heavily that they can no longer kill gram positive. They are beta lactamase resistant though.
|
|
|
What are the two types of glycopeptide antimicrobials?
|
Vancomycin and teichoplanin
|
|
|
What are the hydrophilic/hydrophobic properties of glycopeptides?
|
large and lipophilic
|
|
|
What does this mean for what it can affect?
|
Can only do gram positive because the capsule layer of gram negative are both hydrophilic and phobic in areas.
|
|
|
What are the 6 classes of antibiotics that work by inhibiting protein synthesis?
|
1. Aminoglycosides
2. Tetracycline 3. Macrolides 4. Clindamycin 5. Chloramphenicol 6. Oxazolidonones |
|
|
Where will aminoglycosides bind?
|
Mainly to the 30S of ribosomes to prevent tRNA from forming the inititation complex
|
|
|
Where will newer aminoglycosides bind? Why?
|
to the 50 S to increase the sopectrum.
|
|
|
How are aminoglycosides traported into the cell?
|
Through oxidative phosphorylation.
|
|
|
What are some examples of aminoglycosdies?
|
streptomycin
gentamycin (usually of choice) neomycin |
|
|
How do resistance mechanisms to aminoglycosides work?
|
Enzymes which would phosphorylate, adelylae, or acetylate the drugs to inactivate them.
|
|
|
Describe how tetracyclines work.
|
They bind reversibly to the 30S and do what the aminoglycosides do except they are bacteriostatic.
|
|
|
What can tetracyclines get into that aminoglycosides cannot? WHat does this result in in cildren?
|
It gets into teeth and bones. It used to stain them in children so we don't use them anymore for them.
|
|
|
What is the main example of a tetracycline?
|
Doxycycline.
|
|
|
How do macrlides work?
|
they bind to the 50S ribosome to block translocation.
|
|
|
What are two examples of macrolides?
|
Erythromycin and Azithromycin (Z-oack)
|
|
|
Describe chloramphenicol.
|
Binds to 50S ribosome
|
|
|
Why don't we use it in the US?
|
It is kind of toxic and inhibits the synthesis of all sorts of blood cells (WBC and RBC). However, it is cheap to make and is prevalent in 3rd world countries.
|
|
|
What is a special propoerty of the penetrance of chloraphenicol?
|
It can penetrate our cells and get to intracellular microbes like rickettsiae.
|
|
|
Describe how clindamycin works.
|
Binds to 50S subunit similar to the macrolides, but has extended spectrum to the gram negaive anaerobes.
|
|
|
What is a negative side effect of clindamycin?
|
The proliferation of C. difficile, leading to diarrhea
|
|
|
Describe Oxazolidonones.
|
It is the most recently approve antibiotic which binds the 50S reversibly. It is active against gram positives that are resistant to other agents.
|
|
|
What is an efflux pump?
|
A pump that bacteria develop to pump out the antibiotics.
|
|
|
What are the 4 classes of antibiotics that inhibit nucleic acid synthesis?
|
Quinolones
Metronidozole Rifampin Folate inhibitors |
|
|
How do quinolones work?
|
They inhibit DNA gyrase through topoisomerase II
|
|
|
How do bacteria resist quinolones?
|
mutations in the gyrase
|
|
|
What does the addition of flouride to quinolone to made fluoroquinolone do?
|
It increases their absorption, but it also has many negative side effects
|
|
|
What are two examples of fluoroquinolones?
|
Ciprofloxacin and Levoquin
|
|
|
What does metronidozole do?
|
It introduces SS breaks in the DNA
|
|
|
How is metronidozole delivered and what happens to it to become active?
|
It is delivered as a prodrug and will ponly be redyuced to be active in the presence of anerobes and protzoans.
|
|
|
Describe rifampin function
|
Binds to the b-subunit of RNA polymerate to inhibit transcription
|
|
|
What is the only organism that rifampin is used for? Why?
|
mycobacterium tuberculosis because it has a very slow replication rate and needs to have antibiotics given over a long period of time for it to die out. (doesn't really make sense)
|
|
|
Describe folate inhibitors. Why is it an absolute antibiotic?
|
Folate is necessary for the synthesis of DNA nucleotides for bacteria. We get all our folate from dietary sources so we aren't affected.
|
|
|
What is an example of an antibiotic that is a cell membrane disrupter? Why don't we use it in the US?
|
Polymixins. They are toxic to our cell membranes as well.
|
|
|
When does penicillin work the best?
|
WHen the cell is growing.
|
|
|
When is it not good to use penicillin then?
|
When you are inhibiting protein synthesis because there is little growth
|
|
|
Do you ever use antagonistic antibiotics?
|
Yes. When you need to overcome resistance.
|
|
|
What is intrinsic drug resistance?
|
When the drug is not toxic due to some intrinsic characteristic of the bacteria.
|
|
|
What is the intrinsic resistance of mycoplasma?
|
They don't have a cell wall which means that they are intrinsically resistant to all cell wall antibiotics.
|
|
|
What is the intrinsic resistance of anerobes?
|
They do not have oxidative phosphorylation so are resistant to aminoglocosides.
|
|
|
Where do intrinsic resistances from from?
|
The chromosome of the bacteria
|
|
|
What is the intrinsic resistance of staphylococcus?
|
They produce betalactamase from their chromosomes
|
|
|
WHat are the two types of acquired resistance?
|
1. Mutational change
2. Plasmid tranmitted from another organism |
|
|
How is the fidelity of DNA polymerase in bacteria? What ceonsequence does this have?
|
Not that welll. This means that they can mutate quickly.
|
|
|
What are R plasmids?
|
Plasmids that transmit resistance genes.
|
|
|
What is conjugation?
|
When you transfer a plasmid from one bacteria to another
|
|
|
What is transposition?
|
When a gene from a plasmid jumps onto the chromosome
|
|
|
What are three mechanisms for reisstance?
|
1. Altered transport (exclusion)
2. Altered target 3. ENzymatic inactivation of the antibiotic |
|
|
What is included in altered transport?
|
Efflux pumps and also barriers to entry
|
|
|
What does clavulanic acid do? What does it look like?
|
It is also a beta lactam and will take the hits from the beta lactamase while binding with it to inactivate it.
|
|
|
What is the best example of a combination with a beta lactam drug with calvulanic acid?
|
Augmentin- amoxicilin with clavulanic acid?
|
|
|
What kind of resistance did MRSA develop?
|
It altered the target. It has an altered penicillin binding protein that no longer binds penicillin.
|
|
|
What kind of resistance did vancomycin resistant enterococcus develop?
|
It adds an extra lysine AA to the end of the peptide so that vancomycin cannot bind.
|
|
|
How is resistance to ribosomal antibiotics created commonly> |
Production of new enzymes that methylate an adenine in the 23s rRNA preventing antibiotic binding. |
|
|
why is it so important to study antibiotics above the other drugs (anti viral, anti fungal, anti parasitic, anti cancer)? |
they have been responsible for the most improvement in human survival
viral- hides in human cells, hard to target fungal- eukaryotic so kills us as well parasite- we don't have much of them? cancer- can't selectively target our own cells |
|
|
what is the idea behind bacteristatic drugs? |
stop the growth and let the bacteria die or the immune system handle the rest |
|
|
what kinds of drugs to give immunocompromised pt? can you always do this? |
bacteriocidal- no because a lot are resistant and must have bacteristatic |
|
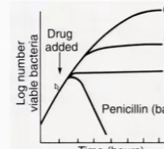
explain |

|
|
|
where can drug resistance arise? 3 |
1. bacteria hides in place not accessible (biofilm) 2. covers up transporter to bring it in 3. mutates binding targets |

