![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
|
What molecule is a polar solvent and tends to interact better with polar molecules
|
WATER
|
|
|
What 2 properties make amino acids, proteins, carbohydrates, and nucleotides soluble in water but not in “lipid” solvents
|
1. are highly polar
2. ionized |
|
|
hydrophilic
|
soluble in polar (water) solvents
insoluable in non polar (lipid)solvents |
|
|
lipophobic
|
insoluable in water
soluble in non-polar solvents |
|
|
Amphipathic molecules
|
contain both hydrophobic and hydrophilic portions in their structures.
|
|
|
Examples of amphipathic biomolecules
|
pulmonary surfactants, bile acids and phospholipids
|
|
|
How does soap function as an amphipathic substance in industry
|
* Detergent action
– the nonpolar R groups in the soaps bind to lipids in the skin or clothing, the ionized carboxyl group can pull this bound lipid into the water phase |
|
|
Why and how a detergent can kill germs?
|
Lyses the cell membrane and destroys the lipid bi-layer;
|
|
|
Why bleach and alcohol can kill bacteria and viruses?
|
Alcohol is amphipathic.
Bleach contains free radicals which kill micro-organisms |
|
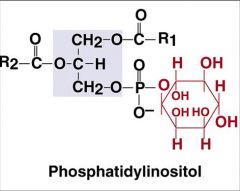
Structure
|
phospho-lipid
|
|
|
Structural confirmation and function of biological membranes:
|
a.Non-covalent assemblies of proteins, lipids, with carbohydrates in a sheet-like structure
b. function as transporters, channels, enzymes, & signal transducers |
|
|
Oligosaccharide chains are attached at outer face of lipids or proteins. What is their purpose?
|
Energy transport and cell membrane operation
|
|
|
The plasma membrane core contain what percentage of cholesterol, phospholipids and sphingolipids.
|
1/3 cholesterol
2/3 phospholipids / sphingolipids |
|
|
The outer leaflet contains glycolipids in what percentage.
|
5% glycolipids
|
|
|
Sphingomyelin and phosphatidylcholine are mainly where in the bilayer?
|
the outer face
|
|
|
Where are Phosphotidylethanolamine and phosphatidylserine found?
|
the inner face of the membrane
|
|
|
Name 2 membrane glycolipids
|
cerebrosides and gangliosides
|
|
|
TRUE OR FALSE
Membrane lipids spontaneously form bilayers? |
TRUE
|
|
|
What molecules make up the highly polar head of the phosolipid molecule?
|
phosphate, choline, and serine groups of the phosophoglycerides
|
|
|
What promotes lipid bilayer from a gel (crystalline) state to fluid state?
|
HEAT
|
|
|
What factors affect the fluidity of membrane?
|
Anesthetics
Alcohol Cholesterol content Degree of saturation Heat |
|
|
Amphipathic lipids making up the cell membrane are stabilized by what?
|
hydrophobic interactions
non-covalent bonds Van der Waals |
|
|
Which is easier for membrane components? Lateral or vertical (flipping) movement and why?
|
lateral
Flipping movement requires hydrophobic components to interact with the lipid area of the bilayer and a transporters (flippase) |
|
|
Name the 2 main functions of the biological membrane.
|
1. Permeability barrier
2. Solvent for membrane proteins |
|
|
Functions of membrane lipids
as a permeability barrier? |
a. selectively permeable-keeps inside and outside seperate
b. maintains homeostatis c. creates compartments within cells |
|
|
Functions of membrane lipids as a solvent for membrane proteins to perform other functions:
|
(1)Regulate cell volume
(2)Maintain intracellular pH (3)Selectively regulate ionic composition (4)Concentrate metabolic fuel |
|
|
An example of how membranes selectively regulate ionic composition.
|
Sodium-potassium pump
|
|
|
An example of how membranes concentrate metabolic fuel.
|
Glucose by glucose transporters
|
|
|
Cellular fraction of the blood
1. components 2. what percentage of blood volume |
a. Erythrocytes (RBC)
Leukocytes (WBC) Thrombocytes (platelets) b. 40 - 45% |
|
|
Noncellular fraction of the blood
|
Plasma & serum
|
|
|
Major components of plasma
|
Water
Protein Other |
|
|
Which cell makes up most of the cellular fractions cell mass
|
RBC
|
|
|
2 categories of Leukocytes
|
Granulocytes
Agranulocytes |
|
|
Types of Granulocytes (PMN's)
|
Neutrophil
Basophil Eosinophil |
|
|
Types of Agranulocytes
|
Lymphocyte
Monocyte |
|
|
Fragments of megakaryocytes
|
Thrombocytes/Platelets
|
|
|
Serum
|
the overlying extracellular fluid in blood after clot formation
|
|
|
Plasma
|
In vitro- the overlying extracellular fluid portion when an appropriate anticoagulant is added to blood
In vivo – extracellular fluid in the blood |
|
|
The chemical components present in the plasma, but not serum, are:
|
(1)Fibrin precursor (fibrinogen)
(2)Added anticoagulant (3)Unused expendable clotting factors |
|
|
Major components of plasma
|
a.Water (about 93%)
b.Proteins (7% ) [albumin, globulins, and fibrinogen] c.Smaller m.w. materials (> 0.1%) [Anabolic constituents, Catabolic products, Electrolytes, hormones, vitamins, and other] |
|
|
Why are lipids not counted in the plasma concentration?
|
Lipids are dissolved into blood concentration but not counted in the plasma concentration bc metabolized and incorporated into globulins by lipoprotein.
|
|
|
Majority of serum protein is produced by what organ?
|
Liver
|
|
|
Reasons for decreased cellular fraction
|
-Increased destruction
-Non-replacement of cells |
|
|
Reasons for increased cellular fraction
|
- increased cells numbers
- decreased serum/plasma concentration |
|
|
Agitation has more or less effect on WBC verses RBC? Why?
|
-less
-Wbc cytoskeletons are more flexible and can take more pressure/agitation than rbc |
|
|
Explain how the degree of saturation affects membrane fluidity?
|
Cholesteral is composed of long chains of carbon and R groups such as oxygen and hydrogen. If a molecule is fully saturated it has all single bonds between the carbon and R groups. These bonds have very limited flexibility. Double or triple bonds present express degrees of unsaturation and allow for rotation around the bond. Bond rotation increases fluidity.
|

